In this October 2017 issue
- Implementation update: Statistics from live jurisdictions
- System update: New functions now available
- BloodSTAR survey: Response overview
- Adverse event reporting: How to report an incident
- User Reference Group: Call for interested parties to be involved
- Ig Governance update: Subscribe to our new webpage to receive Ig updates
- Logon details reminder: Do not share your logon details
- Previous editions
- Download BloodSTAR News
 Pdf (453.27 KB)
Pdf (453.27 KB)
Implementation update
As of October 2017 there are:
- Just over 8000 patients with active authorisations, and
- Approximately 9000 registered users accessing BloodSTAR as Authorisers, Medical Officers, Nurses, Admin support or Facility Administrators.
During September there were 710 initial authorisation requests and 9516 dispense episodes of IVIg/SCIg in BloodSTAR nationally.
System update
The National Blood Authority (NBA) continues to work with users to ensure the system is fit for purpose. On 27 August 2017 an updated version of BloodSTAR (version 2.6) was released with new functions and enhancements. This release included:
Medical Officers
- Dose change – A dose change can now be submitted for conditions in the category of ‘exceptional use only’ (custom dose).
- Initial authorisation request – Link to AHPRA website is now visible when requesting a new Treating Medical Specialist who is not yet registered in BloodSTAR.
- New initial authorisation request – additional information to make notifications clearer on how to proceed when a patient already has an existing authorisation.
Nurse/Midwife/Admin Support
- View authorisation – New ‘Assessment Amendment History’ tab detailing dose amendments by the Authoriser (also available to medical officers and authorisers).
- Dose request – Removed ability to create and submit dispense requests for expired authorisations.
- Planning sheets – ‘Submit button’ has been moved and a clear message is now displayed when a planning sheet is still in draft.
Authoriser/Authoriser Administrators
- Patient record – New ‘Legacy Authorisation’ panel displaying the original legacy authorisation details.
- Edit dose function – Enabled for all dose types only where there are no dispenses, dispense requests or un-assessed authorisation requests.
- Assessment –‘Preview treatment plan’ showing existing doses in addition to the new planned doses
Survey responses
In June 2017, the NBA conducted a survey to get feedback from all users of BloodSTAR and response was received as follows:
- Facility administrators – 76
- Authorisers – 20
- Nurses – 113
- Medical Officers – 62
Feedback indicated that users have had an overall positive experience with BloodSTAR and that there are some improvements to the system that should be implemented to enhance user experience. Below are responses to some of the survey questions:
How would you rate your overall experience of Blood STAR?
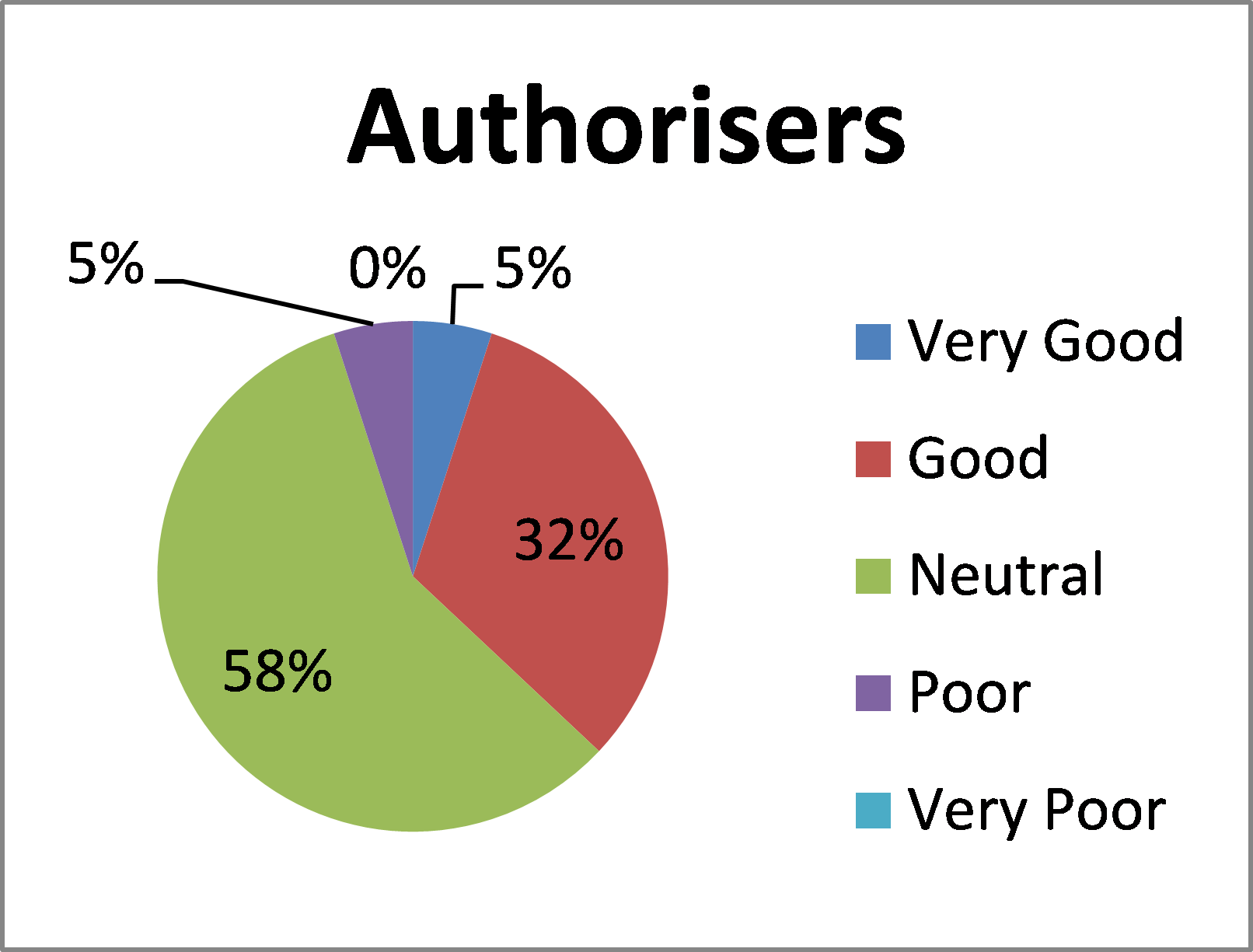
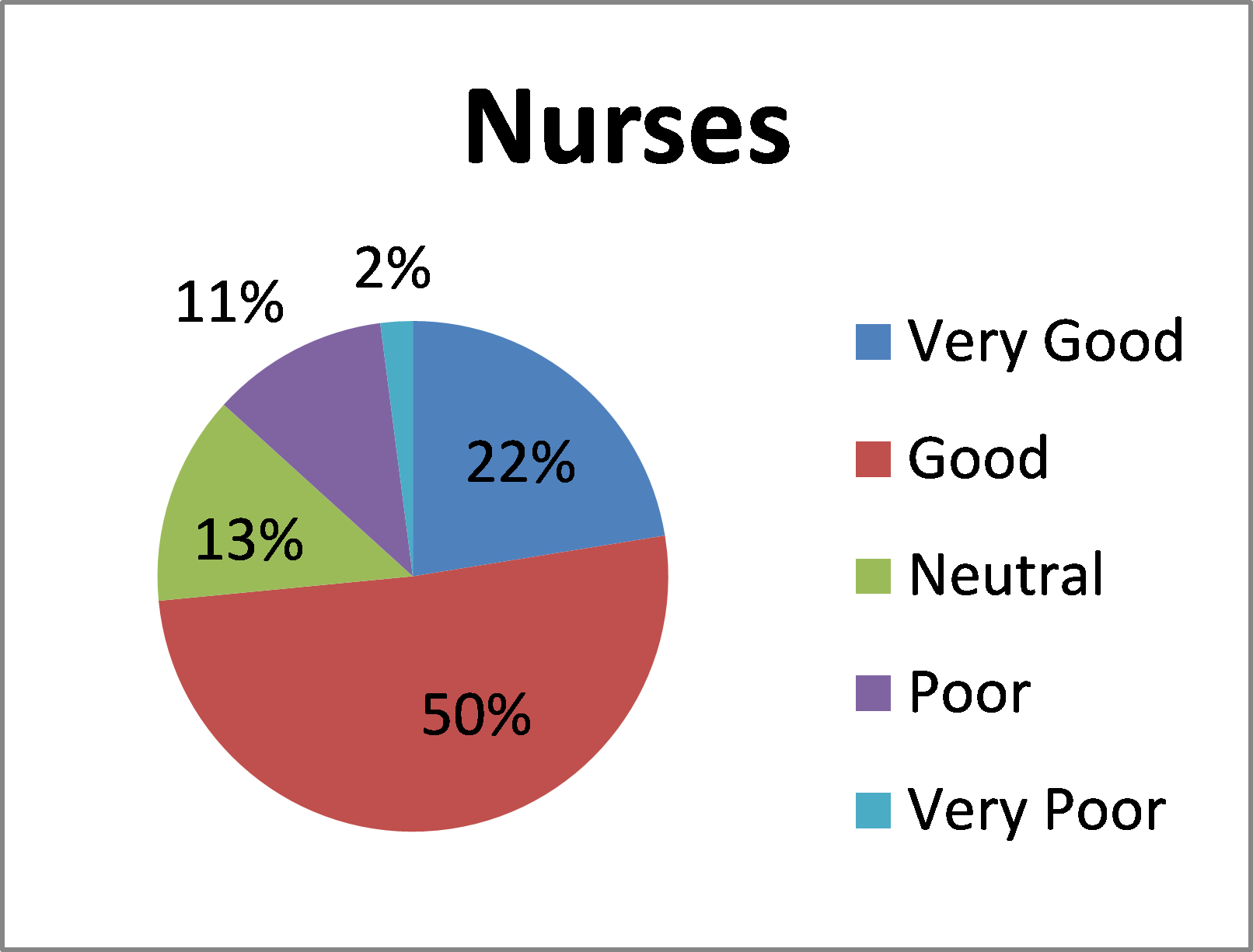
How does the timing for actioning access requests in BloodSTAR compare to your expectation?
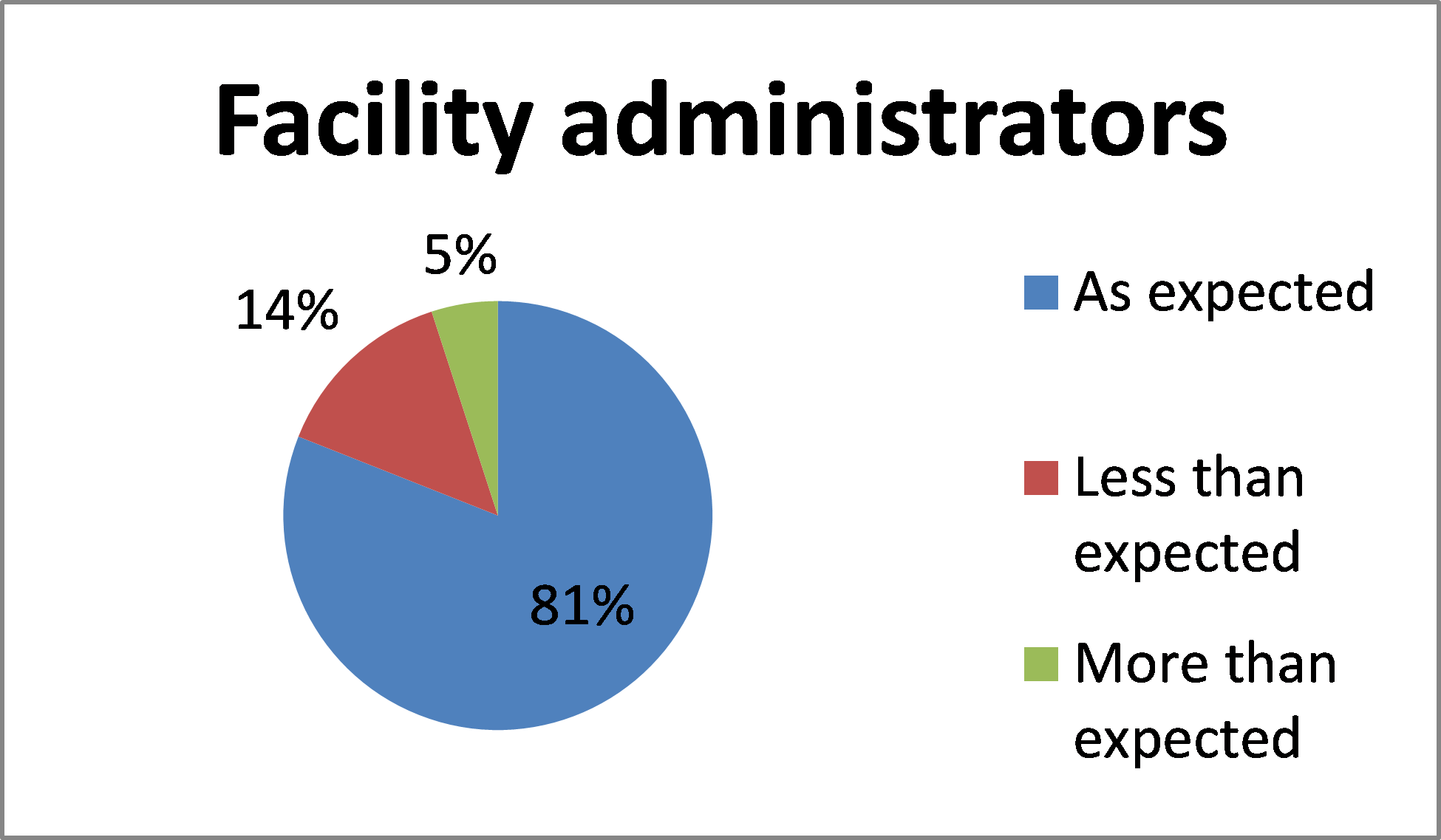
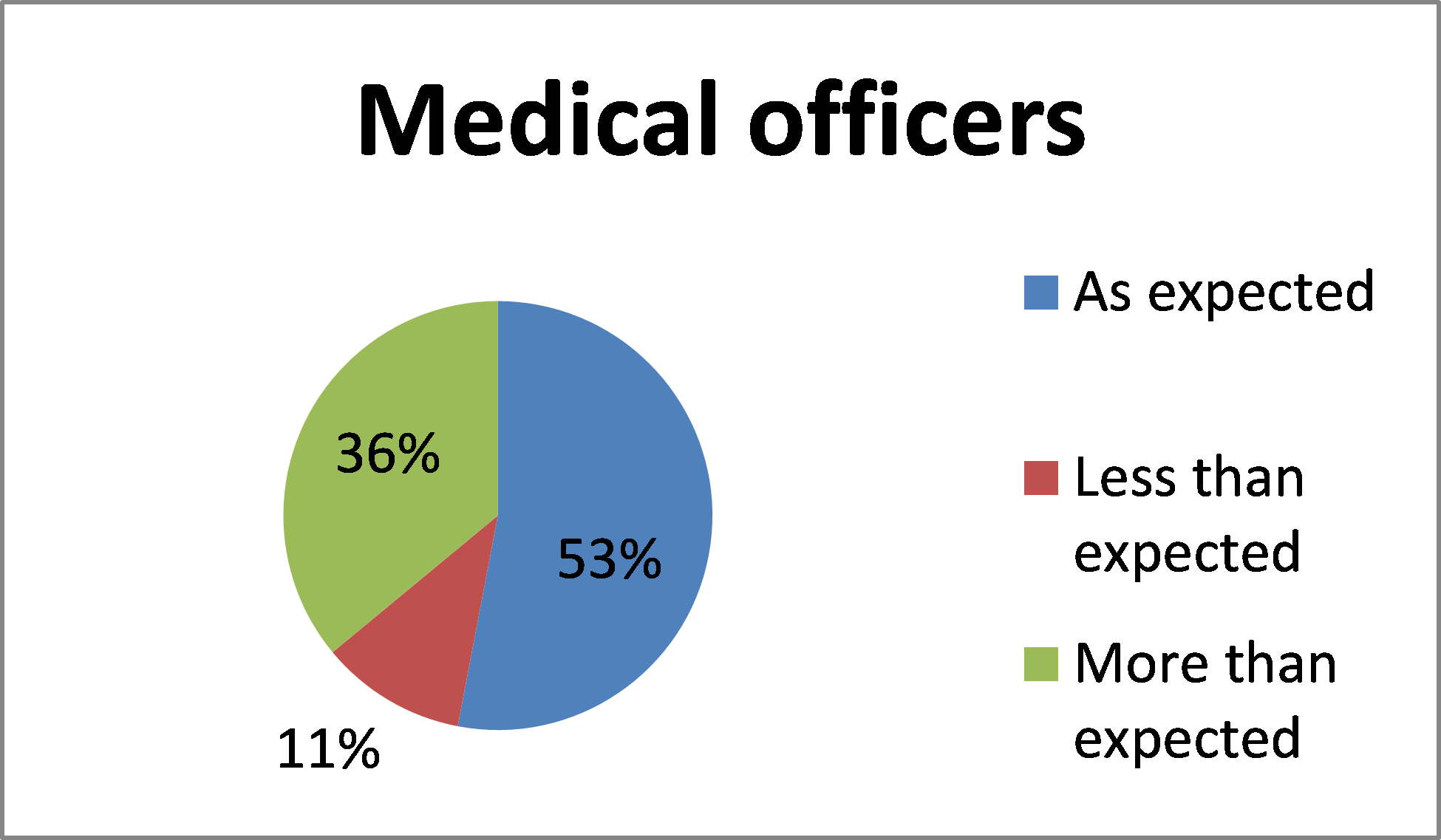
How does the timing for submitting an authorisation request in BloodSTAR compare to your expectation?
Comments collected from respondents have been taken into consideration and helped inform activities for the BloodSTAR working group. We will be updating you as change requests and suggestions are implemented in the upcoming system releases.
Adverse event reporting
If you need to report an adverse event you should follow your local policies and procedures for incident reporting. In addition, you should report the event to the supplier and the Therapeutic Goods Administration directly.
Alternatively, you can also contact the Blood Service to report the incident. They will be able to provide you with the necessary forms to report the incident to the supplier and can submit the completed forms to the supplier on your behalf.
BloodSTAR has the ‘Do Not Prescribe’ functionality in each patient’s record, which is designed to prevent the system from allocating that product to the patient in the future. It is important to note that BloodSTAR is not an adverse event reporting tool, and this function is not used to report the reaction to the Blood Service or supplier.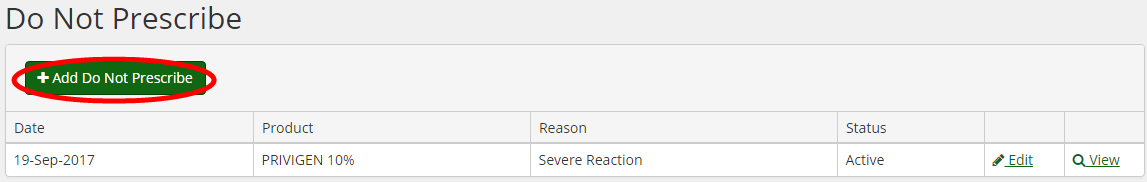
User reference group
We would like to establish a BloodSTAR User Reference Group. The main aim of this group is for the NBA to obtain ongoing feedback and input in relation to updates and proposed changes to the system from users of BloodSTAR across Australia.
We aim to meet face to face annually and quarterly via teleconference with email discussions in between these meetings. We are therefore seeking membership from users who may be interested. If you would like to participate please let us know by providing your full name, facility name, position, contact phone number and email address to support@blood.gov.au by Friday 27 October.
Ig Governance Update
Keep up to date with all the latest Immunoglobulin Governance updates by subscribing to our Ig Governance Criteria and Progress Updates webpage.
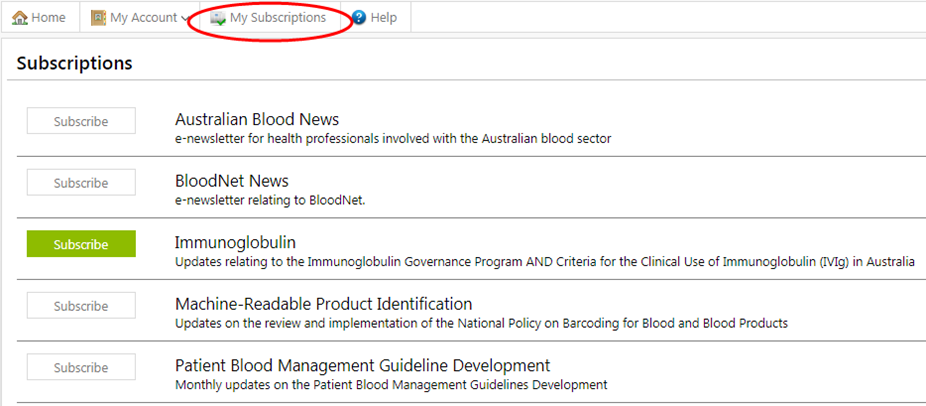
All BloodSTAR users will receive notifications when they are available. If you are not a BloodSTAR user, you can subscribe by creating a BloodPortal account and from the home page click on ‘My Subscriptions’ and ‘Subscribe’ to Immunoglobulin. The latest update is available at Ig Governance Criteria and Progress Updates.
Logon details reminder
Please do not share your logon details with anyone. This is very important to ensure you comply with Australian Privacy Principles and the Privacy Act 1988. As stated in the BloodSTAR User Terms and Conditions, you “must keep logon details secure and not disclose them to any other person.” As a BloodSTAR user you are required to comply with these Terms and Conditions, failure to comply may result in your access being revoked. The Terms and Conditions are available on your BloodSTAR home page.
If you need assistance with creating your own Blood Portal account or updating your current role and facility access to BloodSTAR, please call 13 000 25663 for support or click here to access support material.
For further information
Further information on BloodSTAR is available online at www.blood.gov.au/bloodstar or by contacting the NBA on 13 000 BLOOD (13 000 25663) or support@blood.gov.au.
Previous editions
To read or download previous editions of BloodSTAR News, click on the relevant link below:
2017
2016
- BloodSTAR News December
- BloodSTAR News November
- BloodSTAR News August
- BloodSTAR News June
- BloodSTAR News May
- BloodSTAR News April
- BloodSTAR News March
- BloodSTAR News February

