In this Winter 2019 issue:
- System Activity Update
- User Tips
- National Patient Search
- Requesting multiple dose types during and after initial authorisation
- Ig Governance Update
- Download BloodSTAR News
 pdf (294.42 KB)
pdf (294.42 KB)
System Activity Update
As of 22 July there were:
- 13,834 patients with active authorisations, and
- 13,469 registered users accessing BloodSTAR as Authorisers, Medical Officers (Prescribers), Nurses, Admin Support and Facility Administrators.
In June 2019, there were 1,004 initial authorisation requests and 17,366 dispense episodes of IVIg/SCIg in BloodSTAR nationally.
User Tips
National Patient Search
BloodSTAR allows the roles of Medical Officers (Prescribers), Nurses, Admin Support and Authorisers to view a patient’s record at another facility and/or state. This may be required for travelling patients or those transferred from another facility.
1.Click the ‘Patient’ tab and select ‘Search’ from the dropdown.
2.Enter in the patient’s authorisation number or at least two search terms (given name and family name are deemed as one search term).
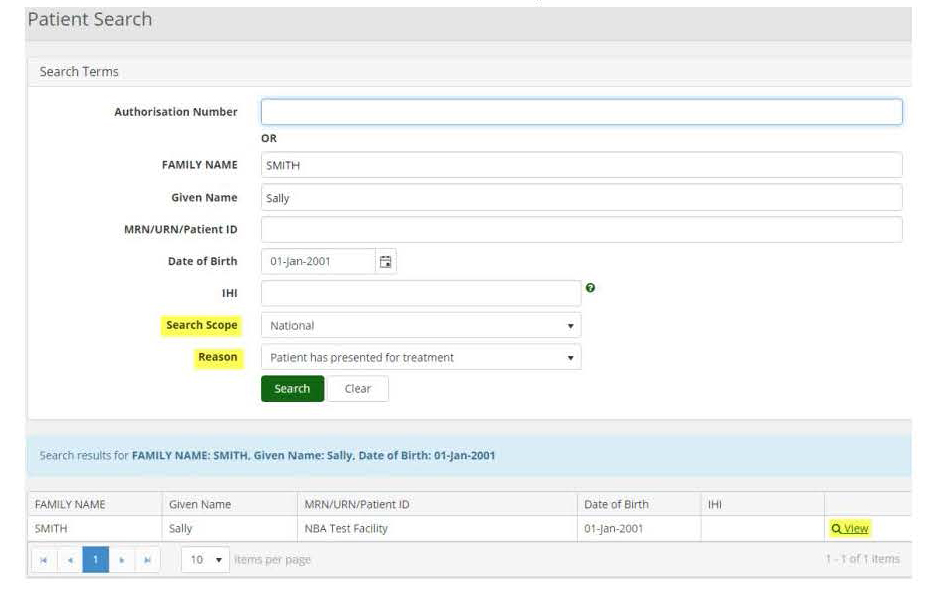
3.Select the search scope (state or national), and a reason from the dropdown, then click ‘Search’.
4.This will bring up the patient’s record which can be accessed by clicking on the ‘View’ link beside their name.
Requesting multiple dose types during and after initial authorisation
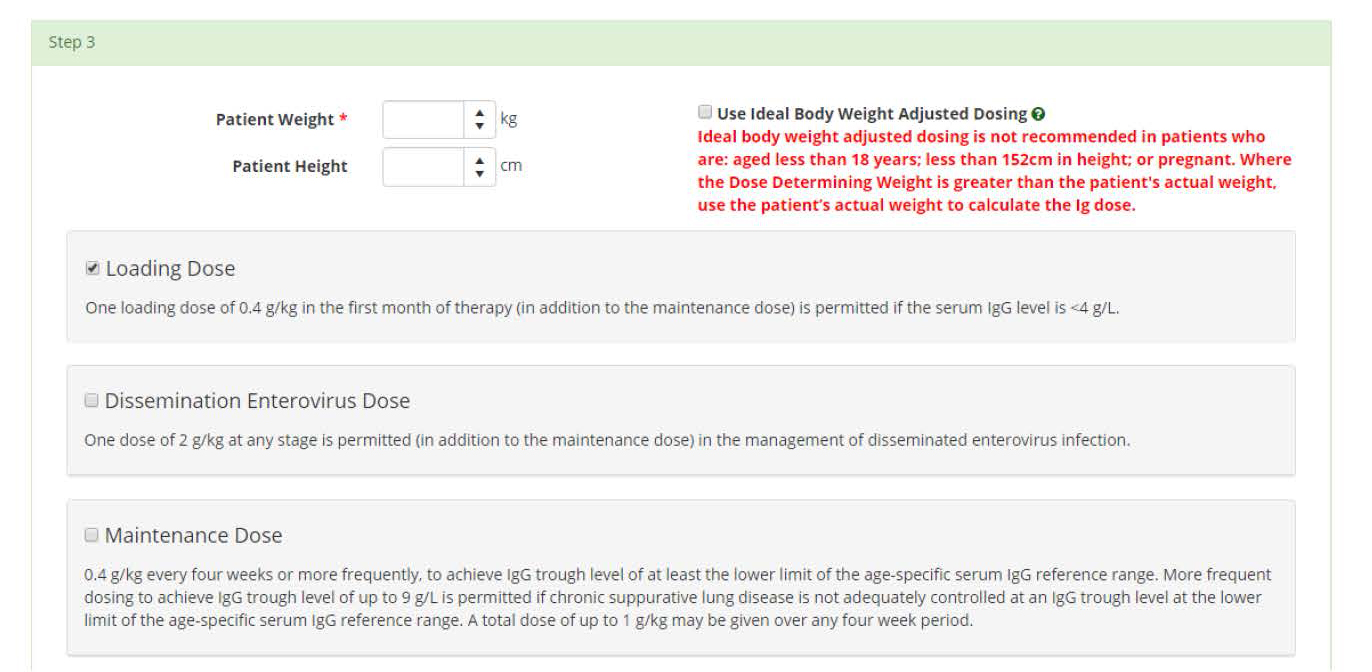
When requesting an initial authorisation in BloodSTAR, certain medical conditions allow the Medical Officer (Prescriber) to request more than one dose type at a time, such as a loading dose and a maintenance dose. When filling in the dose section of the authorisation request, the system will automatically display all doses available for request by the Prescriber.
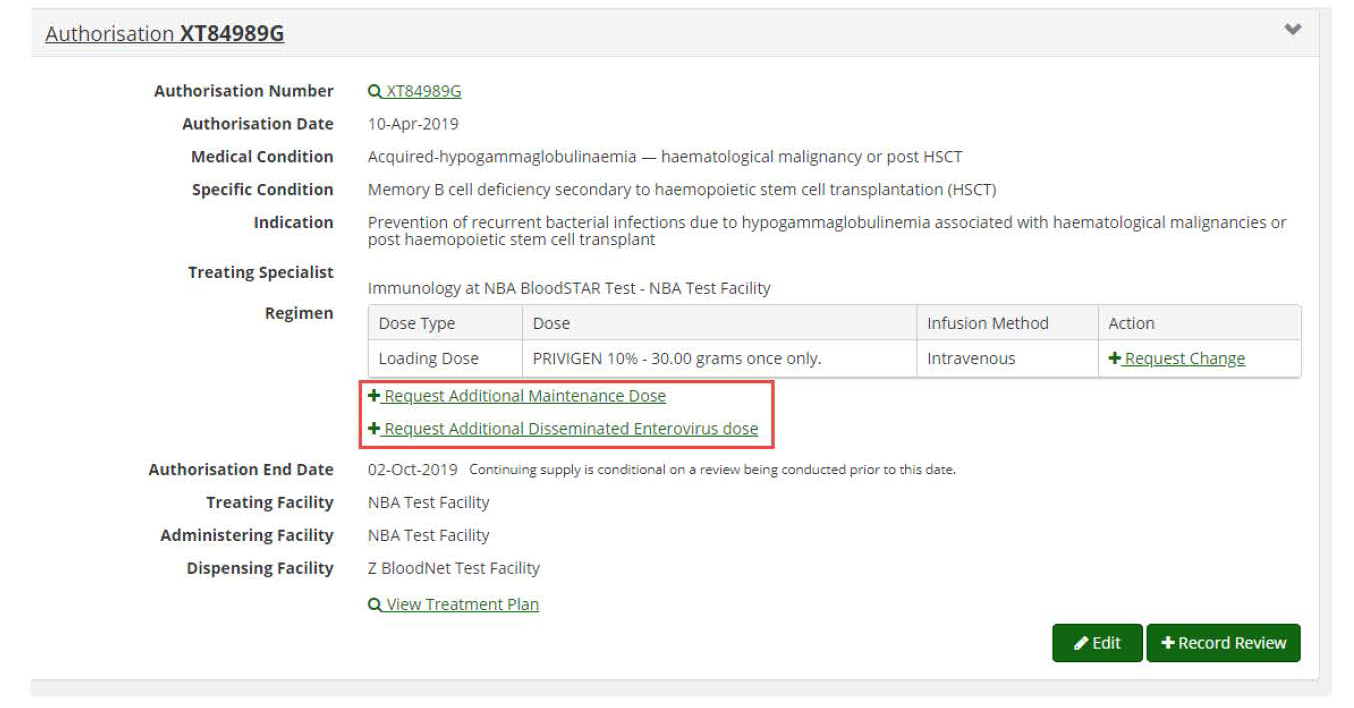
If the medical condition allows the Medical Officer (Prescriber) to request multiple dose types (e.g. a loading dose, maintenance dose and disseminated enterovirus dose) in the initial authorisation but only one is requested, the other dose types can still be requested after the initial request is approved. These other doses will be available to be requested under the current authorisation section of a patient’s record, as shown below.
Ig Governance Update
National Policy: Access to Government Funded Immunoglobulin Products in Australia (National Policy)
The National Policy sets out the process that must be followed, and describes the rules and requirements that must be complied with, to access government-funded Ig products in Australia.
The National Policy has been revised with effect from 15 July 2019. The update takes into account feedback from stakeholders, clarifies policy objectives and provides additional information to further support the latest release of BloodSTAR and Version 3 of the Criteria. The update does not change arrangements for access to government-funded Ig products.
The latest edition of the National Policy is available here and replaces all previous editions.
Access to SCIg for CIDP
From 1 August 2019, subcutaneous immunoglobulin (SCIg) will be available for chronic inflammatory demyelinating polyneuropathy (CIDP) under the national blood arrangements.
This arrangement will be in place pending the outcome of a current Health Technology Assessment (HTA) review evaluating the use of immunoglobulin in the treatment of CIDP. The outcomes of the HTA review will inform more permanent arrangements. Further information about the HTA review is available here.
For further information

Further information on BloodSTAR is available online here. We welcome feedback and suggestions on how we can improve this newsletter. If you have any topics you would like included in future newsletters, please let us know by calling 13 000 BLOOD (13 000 25663) or by sending an email to support@blood.gov.au.
Previous Editions
To read or download previous editions of BloodSTAR News, click on the relevant link below:


