Access to Subcutaneous Immunoglobulin (SCIg) [1]
- Overview: Access to SCIg in Australia
- Supply Under National Blood Supply Arrangements
- Supply Outside of National Blood Supply Arrangements
- SCIg Support Materials
- Access to Other Immunoglobulin Products [2]
Overview: Access to SCIg in Australia
There are two main ways subcutaneous immunoglobulin (SCIg) is available in Australia:
- Supply Under National Blood Supply Arrangements
- Supply Through Other Arrangements: Jurisdictional Direct Orders; private direct order.
Supply Under National Blood Supply Arrangements
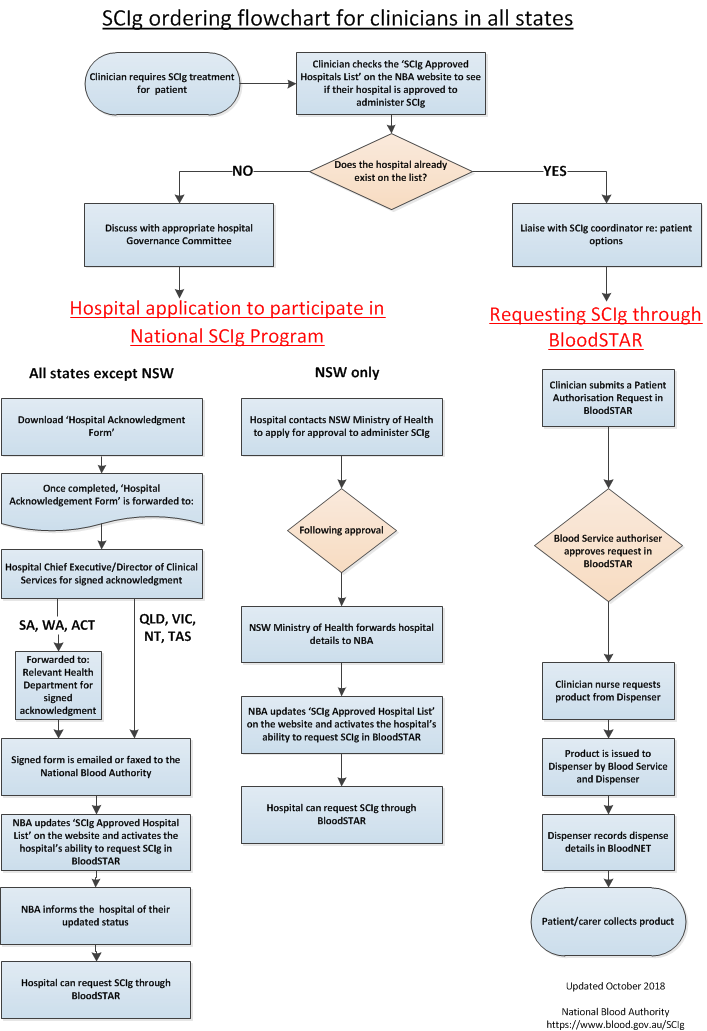
On 1 March 2013, the Jurisdictional Blood Committee (JBC) approved the introduction of subcutaneous immunoglobulin (SCIg) under the national blood arrangements through an assurance framework for the appropriate use of the product.
Participation in the SCIg program requires hospitals to establish their capability and capacity to manage a hospital based SCIg program within the governing requirements described below, and requires endorsement by state health departments in some states. A list of participating hospitals and their contact details is available on this web page.
Approved Access Conditions
SCIg is only available under national blood supply arrangements for patients with a medical condition:
1. where there is support for use cited in the Criteria for the clinical use of immunoglobulin in Australia, namely:
- Primary immunodeficiency diseases with antibody deficiency
- Specific antibody deficiency
- Acquired hypogammaglobulinaemia secondary to haematological malignancies, or post-haemopoietic stem cell transplantation (HSCT)
- Secondary hypogammaglobulinaemia unrelated to haematological malignancies, or post-haemopoietic stem cell transplantation (HSCT)
- Chronic inflammatory demyelinating polyneuropathy (CIDP), (including IgG and IgA paraproteinaemic demyelinating neuropathies)*
* SCIg is approved for use for the treatment of CIDP under the national blood arrangements pending the outcome of a current Health Technology Assessment (HTA) review evaluating the use of immunoglobulin in the treatment of CIDP. For more information please visit here [4].
2. being treated by a clinical specialist within a hospital based SCIg program (see below), where the hospital provides access to all resources and takes full accountability for the management and use of the SCIg product, at no additional cost to patients, and
3. following a patient-specific SCIg request submitted in BloodSTAR.
SCIg Products
The NBA has arrangements in place for the supply of SCIg products. There are both domestic and imported SCIg products available. In seeking authorisation, the requesting medical officer will be asked to provide information to the Australian Red Cross Lifeblood (Lifeblood) through BloodSTAR [5] to establish that the request meets the Criteria. Orders for SCIg for approved patients are made through BloodNet [6].
Access to BloodSTAR [5] and BloodNet [6]is only available through the BloodPortal [7]. To register an individual account follow the process outlined in the instructions and tip sheets available here [8].
Requirements for hospitals participating in national SCIg programs
Hospitals participating in the national SCIg programs are required to provide an acknowledgement of the governing requirements by the Chief Executive or Director of Clinical Services (or equivalent) prior to ordering and providing SCIg products to their patients, using the following form.
In all states (except NSW)
In all states except NSW, hospitals participating in the program are required to provide an acknowledgement of the governing requirements by the Chief Executive or Director of Clinical Services (or equivalent) and forward this form to the National Blood Authority via fax or email (please see contact details below). In South Australia and Western Australia, hospitals participating in the program also require endorsement from their State Health Department.
Please forward the completed form to the National Blood Authority:
Fax: (02) 6151 5300 (Attention: Ig Governance)
Email: iggovernance@blood.gov.au [11]
New South Wales (NSW) requirements
In NSW these requirements are being managed by the NSW Ministry of Health through communication with Local Health District and Specialty Health Network Chief Executives, and this form is not required. For information on NSW arrangements please contact the Office of the Chief Health Officer via email at moh-ocho@health.nsw.gov.au [13].
Hospitals participating in the National SCIg program
A number of hospitals across all states and territories are participating in the National SCIg Program. To view the current list, please click on the image below.
Hospitals participating in the National SCIg Program 2024 (xlsx) [14]
Hospitals participating in the National ScIg Program 2024 (pdf) [15]
Supply Outside of National Blood Supply Arrangements
Where a patient’s condition is not funded under the national blood arrangements, a medical officer may be able to access SCIg products:
- through a jurisdictional direct order (JDO), or
- directly from relevant suppliers on a commercial basis and at private expense.
For further information, see: Ig Access Outside of the National Blood Arrangements [2].
SCIg support materials
The following materials are provided to assist hospital based SCIg programs.
- Generic training checklist for SCIg Program (pdf) [16] (78.88 KB)
- Generic training checklist for SCIg Program (docx) [17] (26.51 KB)
- Patient Information: Subcutaneous Immunoglobulin Treatment (pdf) [18] (636.33 KB)
- Patient Information: Subcutaneous Immunoglobulin Treatment (docx) [19] (2.62 MB)
- Patient Receipt and Use Diary - Subcutaneous Immunoglobulin Treatment Record (pdf) [20](36.33 KB)
- Patient Receipt and Use Diary - Subcutaneous Immunoglobulin Treatment Record (xlsx) [21](12.15 KB)
 Frequently Asked Questions: Subcutaneous Immunoglobulin (pdf) [22] (177.67 KB) (176.34 KB)
Frequently Asked Questions: Subcutaneous Immunoglobulin (pdf) [22] (177.67 KB) (176.34 KB) Frequently Asked Questions: Subcutaneous Immunoglobulin (docx) [23] (148.9 KB) (148.2 KB)
Frequently Asked Questions: Subcutaneous Immunoglobulin (docx) [23] (148.9 KB) (148.2 KB)
Keep up to date
Ig Program Updates [24] provides a snapshot of the NBA’s current work program and priorities in the immunoglobulin space. It is updated quarterly.
To receive the latest Immunoglobulin Governance Program Updates by email, join the Ig Updates and National Immunoglobulin Interest Group (NIIG) subscription list: email Iggovernance@blood.gov.au(link sends e-mail) [25] with the words SUBSCRIBE Ig Program Updates and NIIG in the subject line.
In the body of the email, please indicate your interest (e.g. Healthcare Professional/ Patient/ Government, etc.) and include your signature block.
Subscribers receive notification of the quarterly Ig Program Updates, and may also be invited to informally discuss and comment on individual Ig-related issues as they arise (participation is optional).
For more information on NIIG, see: www.blood.gov.au/Ig-committees [26].
For further information
Please contact the National Blood Authority on 13 000 BLOOD (13 000 25 663) or email IgGovernance@blood.gov.au [27].