3.5 Supply management
'Supply management is not a science. We are dealing with a biological product derived from volunteer donors that is then subject to a complex labyrinth of testing, production and distribution to ensure the required product is available.'
The National Blood Authority is responsible for coordinating Australia's blood supply. The National Blood Authority provides monthly performance reports to jurisdictions on the actual volumes of blood and blood-related products delivered to approved recipients. This underpins the surety of delivery of blood and blood-related products to the Australian community.
National Supply Plan and Budget
The National Supply Plan and Budget development process for 2007–08 commenced in July 2006. The plan was endorsed by the Jurisdictional Blood Committee and approved by the Australian Health Ministers' Conference in March 2007. The National Blood Authority manages the National Supply Plan and Budget in conjunction with jurisdictions, the Australian Red Cross Blood Service and commercial suppliers. Figure 14 sets out the National Supply Plan and Budget planning cycle.
The National Blood Authority worked to improve the processes surrounding the development of the National Supply Plan and Budget in consultation with key stakeholders in both the commercial and government sectors. The Australian Health Ministers' Conference approved the 2007–08 National Supply Plan and Budget at the end of March 2007—the earliest it has ever been approved.
Under the National Blood Agreement, the state and territory governments are responsible for demand management. The National Blood Authority supports the jurisdictions with forecasting and demand modelling through consultations with key stakeholders.
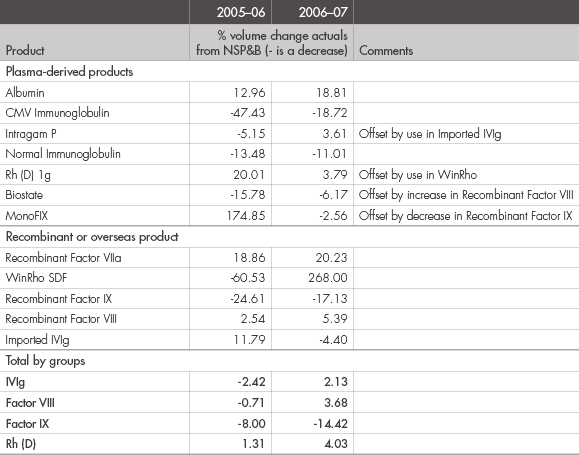
The approved National Supply Plan and Budget for 2006–07 was $639.8 million. The actual demand in 2006–07 was $637.7 million. This represents a decrease of $2.1 million or 0.3 percent—see Figures 15 and 16.
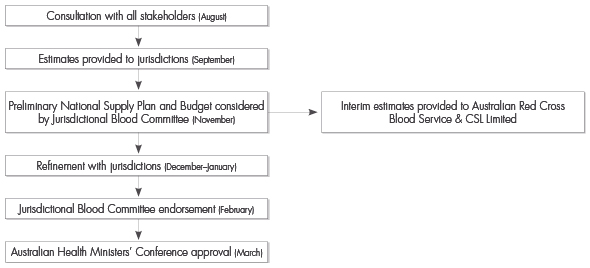
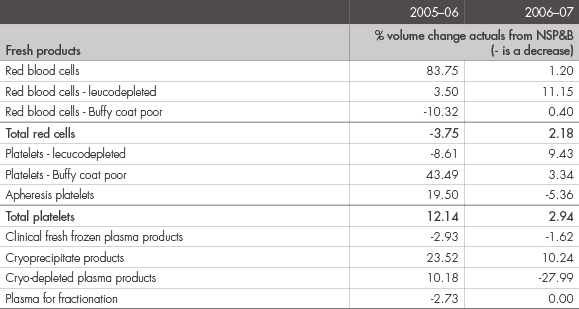
FIGURE 16: VARIATIONS OF PLANNED VOLUMES TO ACTUAL ISSUES OF PLASMA-DERIVED AND RECOMBINANT PRODUCTS
Intensive product management
An ongoing task is to identify required blood product inventory levels, including required reserves and buffers, and to manage contingent order requirements and stockholdings. The nature of the blood sector means that it is, on occasion, necessary to intensively manage products in short supply or for contingent supply arrangements. Intensive management is a core business activity and was successfully undertaken in 2006–07 to avert a number of temporary and longer-term potential shortages, including shortages of IVIg and plasma-derived Factor VIII.
This included the implementation of a range of contingency arrangements. In consultation with key stakeholders, the National Blood Authority implemented protocols for intensive management, distribution and the development of profiles for all products to assist in the setting of inventory target levels to ensure supply.
Development of barcoding policy
The National Blood Authority published a discussion paper on barcoding in the blood sector seeking stakeholder input. Stakeholders' responses were constructive and helped to determine recommendations for a national policy on the barcoding of fresh blood, plasma, recombinant and diagnostic products. The Jurisdictional Blood Committee agreed to the national implementation of ISBT 128 for all fresh blood products and GS 1128 (formerly EN 128) for all plasma, recombinant and diagnostic products by 1 July 2011.

