3.2 Performance indicators
This section reports against the NBA’s results in the 2007–08 reporting period and comments are offered in relation to the performance indicators relative to the output.
Efficient management and coordination of Australia’s blood supply
To ensure efficient management and coordination of Australia’s blood supply, the NBA has supply contracts with various suppliers of blood and blood-related products that it manages closely to ensure that demand for these products is always met. Table 3.2 shows these suppliers.
Table 3.2 Blood and blood-related products purchased in 2006–07 and 2007–08, by supplier
| 2006–07 | 2007–08 | ||
| Supplier | Products Purchased | Amount ($ millions) |
Amount ($ millions) |
| CSL Ltd | Plasma Products – albumin products – immunoglobulin products – Rh(D) immunoglobulin (including IVIg and hyperimmune products) – plasma-derived clotting factors Diagnostic Reagent Products – blood grouping sera – reagent red cell products Defined Blood Products – Factors XI and XIII – Imported IVIg Management of National Reserve |
145.10 | 159.90 |
| Australian Red Cross Blood Service | Fresh Blood Products – whole blood – red blood cells – platelets – clinical fresh frozen plasma – cryoprecipitate – plasma for fractionation |
305.77 | 385.03 |
| Baxter Healthcare Pty Ltd | Defined Blood Products – Recombinant Factor VIII – Protein C – Factor VII concentrate – Factor Eight Inhibitor Bypass Agent (FEIBA) – WinRho |
71.53 | 80.08 |
| Wyeth Australia Pty Ltd | Defined Blood Products – Recombinant Factor VIII – Recombinant Factor IX |
28.88 | 42.38 |
| Novo Nordisk Pharmaceuticals Pty Ltd | Defined Blood Products – Recombinant Factor VIIa |
26.95 | 17.43 |
| Octopharma Pty Ltd | Defined Blood Products – Imported IVIg |
22.24 | 34.26 |
| DiaMed Australia Pty Ltd | Diagnostic Reagent Products – blood grouping sera – reagent red cell products |
0.80 | 0.94 |
| Ortho-Clinical Diagnostics (Johnson & Johnson Medical Pty Ltd) | Diagnostic Reagent Products – blood grouping sera – reagent red cell products |
0.25 | 0.37 |
| Australian Laboratory Services Pty Ltd | Diagnostic Reagent Products – blood grouping sera – reagent red cell products |
0.05 | 0.05 |
| Total Purchases of blood and blood-related products | 601.55 | 720.45 | |
All amounts exclude GST
In 2007–08 the NBA managed 13 blood supply contracts and arrangements. Of these, seven were newly negotiated and none were subject to variation. Table 3.3 shows the new blood product contracts that were negotiated and concluded in 2007–08.
Improvements in price and product quality
The NBA has negotiated contracts with suppliers that have provided recurring annual savings to the jurisdictions for the supply of blood and blood-related products since 2003–04. Savings for 2007–08 are estimated at $26 million based on a comparison of prices that would have been paid under previous contracts and indexation. These savings have been achieved after extensive analysis of benchmark products, close international networking, and consultation with industry analysts. These price savings have been achieved alongside improvements in product quality. People with bleeding disorders have given very positive feedback on the range, quality and service support for the new products. This is reflected in the uptake of these new products — see Figures 3.9 and 3.10.
National administered budget performance
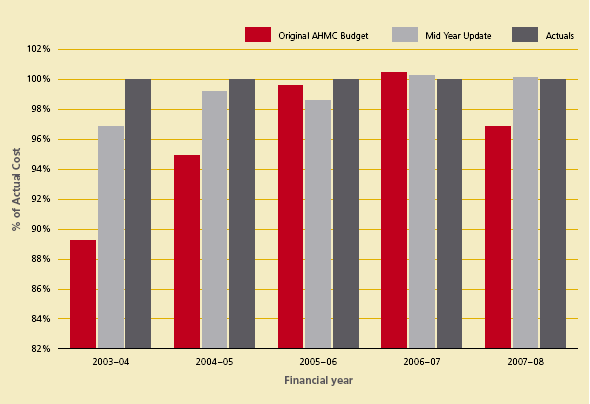
The NBA administered budget is completed annually as part of the National Supply Plan and Budget process agreed by the Australian Health Ministers’ Conference (AHMC). The NBA, in consultation with jurisdictions, reviews the National Supply Plan and Budget annually as part of the mid-year review process to adjust jurisdictional funding requirements as a result of the first six months expenditure. As depicted in Figure 3.1, the NBA’s performance in forecasting and managing the National Supply Plan and mid-year review budget has improved since its inception to 2007–08. In 2007–08, actual expenditure varied by 3% (compared to less than 1% in 2006-07) against the National Supply Plan and Budget and less than 0.2% against the mid-year review. The main driver for the change from the original budget in 2007-08 was increased demand for IVIg, FEIBA and Recombinant Factor VIII.
The budget for 2003–04 was not set by the NBA, as the NBA was not established until 1 July 2003.
Table 3.3 New blood product contracts negotiated, 2007–08
| Supplier | Implementation | Description of Contract | |
| CSL Limited | From November 2007 | For the Supply of Diagnostic Reagents | |
| DiaMed Australia Pty Ltd | From November 2007 | For the Supply of Diagnostic Reagents | |
| Ortho-Clinical Diagnostics (Johnson & Johnson Medical Pty Ltd) |
From November 2007 | For the Supply of Diagnostic Reagents | |
| Australian Laboratory Services Pty Ltd | From November 2007 | For the Supply of Diagnostic Reagents | |
| Octapharma Australia Pty Ltd | From December 2007 | For the Supply of Overseas sourced IVIg | |
| CSL Limited | From January 2008 | For the Supply of Overseas sourced IVIg | |
| CSL Limited | From January 2008 | Management of the National Reserve of Plasma Products. | |
Figure 3.1 Performance of National Blood Authority: actual funding versus budgets
< Previous | Contents | Next >
