3.3 Management of fresh blood products
The ARCBS collects fresh blood from voluntary donors for the purposes of manufacturing a variety of blood products. These products are used for the treatment of major medical conditions such as cancer; heart disease; stomach and bowel disease; liver and kidney disease; haemophilia; and to treat newborn babies and pregnant women. Donated blood is also used to help people who suffer traumatic incidents such as accidents, burns, and during or as a result of surgery. ARCBS also collects plasma, which is provided to CSL Ltd for fractionation into a variety of products to meet the needs of the health sector.
The NBA manages the relationship with the ARCBS — the sole supplier of fresh blood products in Australia — and is responsible for negotiating and managing the ARCBS Deed of Agreement. The NBA also manages a number of projects involving ARCBS, as well as providing secretariat and project management support for the National Managed Fund (NMF) and the National Blood Arrangements Schedule 4 process.
ARCBS funding and product mix
Funding to the ARCBS increased from actual funding of $327.1 million in 2006–07, to an agreed budget of $370.2 million in 2007–08 (see Table 3.4).
The ARCBS continued to be funded on a grant basis during 2007–08. However, the Australian Red Cross Society Board has indicated its support for the implementation of the output funding regime as defined under the terms of the ARCBS Deed. When implemented, this will mean that states and territories pay only for the products and services they order and receive. It is expected the output funding model will be implemented from 1 July 2008.
The growth in funding reflects increased demand and changes in the product mix for fresh blood products. Figures 3.2 and 3.3 show the changes in product mix for red blood cells and platelets over the past five years.
Table 3.4 Australian Red Cross Blood Service, annual funding commitments, 2003–08
| Year | Amount ($ million) |
% Growth |
| 2003–04 | 247.8 | |
| 2004–05 | 277.0 | 11.8 |
| 2005–06 | 297.7 | 7.5 |
| 2006–07 | 327.1 | 9.9 |
| 2007–08 | 370.2 | 13.2 |
| Total | 1519.8 | (average) 10.6 |
Figure 3.2 Product mix of red blood cells issued by the Australian Red Cross Blood Service, 2003–08
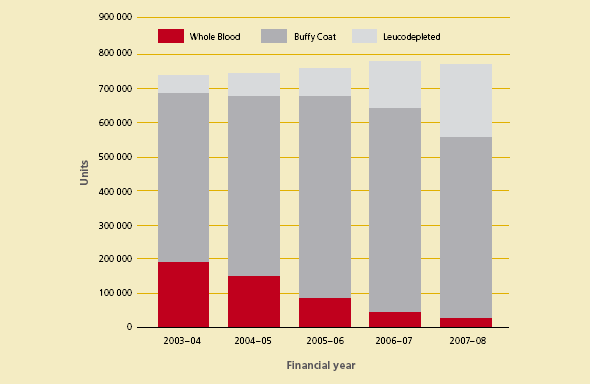
Figure 3.3 Product mix of platelets issued by the Australian Red Cross Blood Service, 2003–08 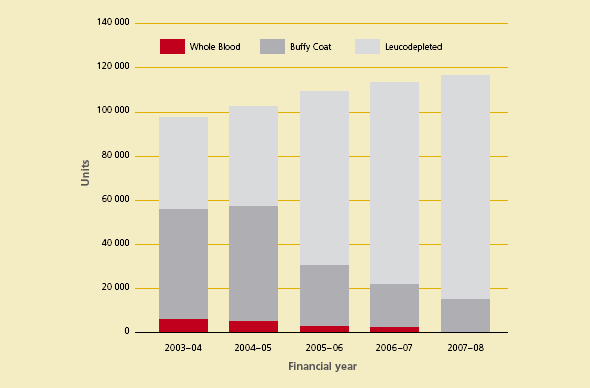
During 2007–08, governments made a number of decisions to give substantial further funding to ARCBS to support the ARCBS principal site developments. Funding was also used to enhance blood safety measures such as leucodepletion, universal prerelease bacterial contamination testing and the introduction of effective donor recruitment and retention measures to meet the Council of Europe’s Guide to the Preparation, Use and Quality Assurance of Blood Components (12th edition) requirements.
ARCBS business study
During the year, the NBA continued to manage the ARCBS business study, which commenced in 2006–07. The expert advisory group (EAG), established to advise the NBA General Manager on the study, met six times from 1 July 2007 to 31 January 2008. Members of the EAG included:
- Mr Michael Roche, Independent Chair
- Ms Kerry Flanagan, Australian Department of Health and Ageing
- Mr Ken Barker, New South Wales Department of Health
- Professor Brendon Kearney, Institute of Medical and Veterinary Science
- Mr Robert Tickner, Australian Red Cross Society
- Dr Robert Hetzel, Australian Red Cross Blood Service
- Dr Philippa Hetzel, Australian Red Cross Blood Service
- Mr Garry Richardson, National Blood Authority Board.
KPMG has provided business consultancy services to assist the study. The business study concluded that, based on the analysis and associated benchmarking, there were many positive aspects of ARCBS’s delivery of the blood service in Australia. The study also found that the ARCBS is striving to improve its services and management.
The business study has, however, pointed out a number of areas for improvement, including the identification of efficiency measures that have the capacity to deliver cost savings in the medium term. A number of these require up-front project funding, but will potentially achieve savings even in the short term. The ARCBS has yet to validate these savings. The NBA will actively pursue progression of these measures with ARCBS, including the identification of possible funding sources for project costs should these be required.
The report and its recommendations are to be provided to the AHMC for consideration at the AHMC July 2008 meeting.
ARCBS Deed of Agreement
During 2007–08, the NBA gave priority to a number of areas of the ARCBS’s operations to ensure that the objectives and intent of the agreement were achieved. This included a focus on ARCBS strategic, capital and business planning; and the assessment of a number of business cases for consideration by governments.
Strategic and business planning framework
The establishment and operation of the Deed framework for providing input to ARCBS’s strategic and business planning processes is not yet fully developed, although it has progressed throughout the year. Successful implementation of this important element of the Deed will ensure that governments’ priorities are clearly articulated and appropriately reflected in ARCBS operations. Priorities that governments expect to be taken into account in this planning process were detailed to ARCBS in April 2008 and include:
- ensuring states and territories only pay for the products and services they request and receive
- engaging with governments in short and long-term planning activities
- progressing and implementing the business study recommendations in line with the agreed response by AHMC at its July 2008 meeting
- developing and implementing an approved health provider customer service model
- implementing improved measures that support retention of existing donors
- contributing knowledge and skills to the blood sector and providing advice to stakeholders through both horizon scanning and the ARCBS research and development program.
Horizon scanning
A core element of this strategic planning is the need for the NBA to strengthen its engagement with the ARCBS in relation to horizon scanning, research and development, and business and capital planning.
With representative membership on the ARCBS Research and Development Committee, the NBA is working with the ARCBS to restructure the research and development program and prioritise research projects. The interest of the NBA in these activities is to ensure decisions to fund particular research and development projects reflect governments priorities.
Figure 3.4 Examples of emerging theme mapping
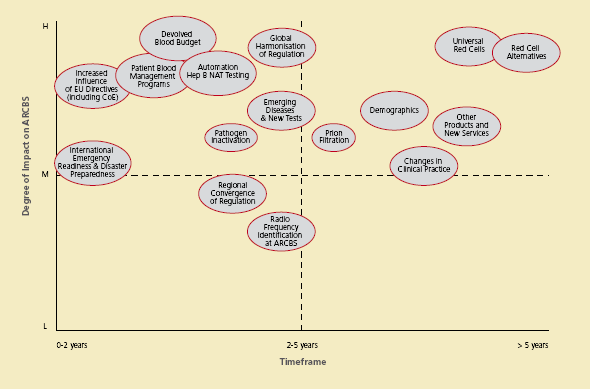
Australian Red Cross Blood Service: The map above illustrates examples of potential emerging themes and developments relevant to the Australian Red Cross Blood Service. The themes are mapped according to their perceived possible degree of impact over a seven year horizon. The ARCBS is continually scanning for themes and clarifying drivers of change.
Under the terms of the Deed there is an expectation that the ARCBS will provide advice to the NBA on new and emerging trends that are likely to affect the provision of blood and blood-related products in Australia, including emerging technologies, public health activities and analysis of international trends in blood banking. In March 2008, representatives from the ARCBS provided an update on its horizon scanning processes and outlined the emergent themes facing the international blood and blood-products sector from their perspective.
The NBA will continue to work with ARCBS on ensuring timely consideration of these issues to ensure careful and balanced decision making for the blood sector.
Strategic capital investment plan
The ARCBS Strategic Capital Investment Plan demonstrates the intended capital expenditure required to sustain fixed assets including asset replacements such as vehicles, laboratory equipments and premises (eg refurbishments, relocations and new sites).
The Strategic Capital Investment Plan covers a five-year period. The first year is the annual capital plan, which outlines the expenditure required for the next financial year. The Strategic Capital Investment Plan is prepared by the ARCBS annually and submitted to the JBC as part of the approval process for the annual supply plan and budget.
Under the Deed, the annual capital funding provided to the ARCBS covers 10% of their main operating program. The value of approved annual capital plans from 2005–08 is shown in Table 3.5.
Table 3.5 Value of Australian Red Cross Blood Service annual capital plan ($ million), 2005–08
| 2005–06 | 2006–07 | 2007–08 |
| 27.02 | 29.58 | 32.96 |
In addition to the approved annual capital plans, governments have agreed to fund, as appropriate, the redevelopment of the principal manufacturing sites to maintain the flexibility and capacity of the ARCBS. This will allow the ARCBS to respond promptly to changing circumstances and needs, and to meet national obligations and standards for maintaining a high quality blood supply in Australia.
An update of the major principal sites is provided below.
Queensland
The ARCBS took possession of its new Queensland principal site, situated within the Queensland University of Technology development at Kelvin Grove on 4 February 2008. The $33.98 million cost of the Queensland facility has been borne by the ARCBS from existing capital funding provided by all Australian governments.
New South Wales and Australian Capital Territory
On 18 April 2008, the AHMC gave policy approval for additional funding of up to $115 million (at 2008 prices) to be provided to the ARCBS to meet building leases and fit-out for a new principal manufacturing site for New South Wales and the Australian Capital Territory.
The new 12,500 m2 manufacturing facility will replace the existing manufacturing facilities at 158 Clarence Street, Sydney and Dann Close, Canberra, which are no longer suitable due to the ARCBS’s growth. When completed in 2009–10, the new facility will accommodate nearly 600 people and contain all ARCBS activities in a purpose-built facility with a proposed minimum occupancy of 20 years.

NBA Board members at their visit to ARCBS Clarance St, NSW facilities
Victoria and Tasmania
During 2007–08, the ARCBS commenced planning for the new Victorian and Tasmanian principal sites. The NBA expects to receive the final business case from the ARCBS in the first quarter of 2008–09, allowing for consideration by the Health Ministers during the second quarter of 2008–09.
Business case assessments
The strategic and business planning processes, established in the Deed, provide for the conduct of feasibility studies and the development of business cases for initiatives that can be shown to meet government and ARCBS future priorities. In 2007–08, the ARCBS submitted the findings of three feasibility studies for the NBA’s consideration. These studies investigated the feasibility of implementing new donor relationship management, a computer-assisted donor questionnaire and improved blood inventory management systems within the ARCBS. The NBA has supported further investigative work on these initiatives with the exception of the Blood Inventory Management System, which requires additional supporting information.
Funding of $1.2 million was approved for release to ARCBS in 2007–08 to address the impact on the blood supply by new requirements on whom can donate blood under the Council of Europe guidelines. The Guide to the Preparation, Use and Quality Assurance of Blood Components (12th edition) is the regulatory standard to which the ARCBS is currently mandated as a minimum standard for fresh blood components by the Therapeutic Goods Administration (TGA). The changes implemented in 2007–08 relate to the management of donors with a history of acupuncture treatment and body piercing. Funding provided will assist in the effective implementation of new donor recruitment and retention measures.
The ARCBS business study required KPMG to review several business improvement initiatives the ARCBS proposed to implement. Work is continuing with the ARCBS to quantify the costs, savings and timeframes associated with implementing these efficiency measures.
Change program funding
As part of the negotiation for the Deed, a change program funding pool of $7 million was established for 2006–07 to 2008–09 to fund the following:
- specific initiatives as part of the ARCBS national change program
- specific transitional initiatives to be implemented under the Deed
- other specific initiatives that will deliver cost savings or otherwise increase the efficiency of the production of goods or services under the deed (only if the purposes of the above two points have been substantially fulfilled by initiatives which have been funded).
As of 27 September 2007, eight of the nine proposals put forward by the ARCBS had been approved by the JBC. Two further proposals had been approved out of session by the JBC and two other proposals had the consideration of their funding withheld pending the ARCBS announcing any other requests for funding. The total funding approved for these ten proposals was $4.6 million. To date, the NBA has received invoices totalling $1.57 million.
ARCBS performance
Engagement between the two organisations is supported via regular communications and meetings, review of key performance indicators and third party reviews.
Communications and meetings
Regular reporting is provided through:
- quarterly meetings between the NBA General Manager and the ARCBS CEO in relation to strategic issues and planning, progress against performance targets and other high-level issues
- Chief Financial Officers meeting quarterly to discuss issues concerning ARCBS financial management and reporting, and capital planning
- regular meetings between the NBA’s deed management team and representatives from the ARCBS regarding matters such as supply planning, intensive product management, contingency planning, principal sites and business cases.
These meetings are an opportunity for an exchange of information and review of ARCBS performance against the Deed. These meetings have seen the approval of a number of protocols and joint arrangements that support effective implementation of the Deed. Processes approved in 2007–08 include public affairs management, acknowledgment of government funding, third party reviews, development and amendments to the Annual Capital Plan, and management of change program funding pool approved projects.
It was also agreed during these discussions that the NBA prepare for ARCBS’ consideration a key document required under the Deed of Agreement, the ARCBS Handover Plan. This document sets out arrangements to apply between the ARCBS and the NBA and any third party in all situations of termination or expiry of the Deed, to ensure the effective management of the blood service (should this ever be necessary).
Key performance indicators
The ARCBS is implementing a number of strategies in the area of donor management. Although faced with a number of challenges in the 2007-08 year (including the impact of cold and flu early in the season) resulting in a decreased number of donors and donations generally, key performance indicator data showed success in implementing these strategies.
The encouragement of regular whole blood donors to donate through apheresis collection of plasma and platelets had a positive impact, evidenced by a rise in the number of apheresis plasma donors and donations in the final quarter of the year.
Development of approaches for increasing donor retention rates (as opposed to increasing the donor panel with first time donors), and increasing the number of donations per donor in the period, are priorities for the ARCBS’s donor management program. Both of these areas showed positive results, with donation frequency higher than planned for whole blood and platelet apheresis donors, and the number of new donors (as a proportion of the total donor panel) decreasing steadily from 28.4% in the first quarter of 2007-08 to 27.1% at the end of the fourth quarter, indicating a slight improvement in the donor retention rate.
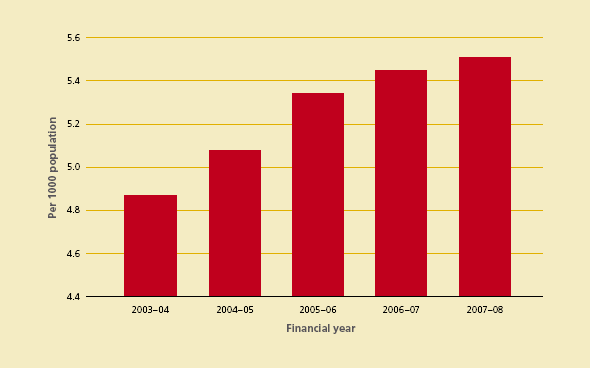
The total demand for red cells in Australia during 2007–08 reduced with a resulting decrease in red cell issuage per 1000 head of population to 36.3 units. (See Figure 3.5). At the same time, platelet issuage per 1000 head of population increased to 5.51 units from 5.45 from the previous year (see Figure 3.6).
Figure 3.5 Red blood cells issued per 1000 head of population
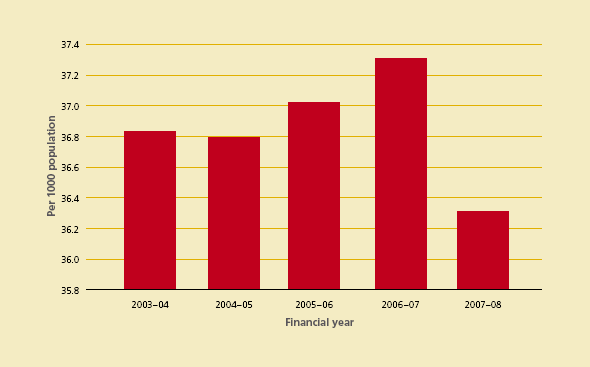
Figure 3.6 Platelets issued per 1000 head of population
Throughout the period, the number of process-related recalls fell below the KPI planning parameter except in quarter 4, during which the introduction of 100% bacterial contamination testing of platelets resulted in a significant increase in the number of recalls.
The NBA and ARCBS have been reviewing the KPIs under the Deed in an effort to improve on quantitative and qualitative reporting. This was done so that the NBA can gain further understanding of the variables contributing to ARCBS performance against the KPIs.
Third party reviews
Third party reviews are jointly commissioned by the NBA and ARCBS as parties to the procurement, evaluation and management of the contract for services. The reviews are designed to assist the two organisations in ensuring that the ARCBS operates in line with better practice in a cooperative and positive framework. The first of the third party review projects under the Deed was tendered for in late 2007–08 on ARCBS governance arrangements. Other third party reviews are to be conducted prior to the current Deed expiry date (June 2009), including the ARCBS’s procurement arrangements; risk management arrangements; and product ordering, delivery, receipt and invoicing.
Agreement to supply project
The agreement to supply project is driven by the reliance of the ARCBS and jurisdictions on the cooperation and good practices of approved health providers to help achieve the most efficient management and use of blood and blood-related products in pathology laboratories and hospitals. This includes integration of planning and demand management, comprehensive reporting on product usage, appropriate storage and inventory management.
The project proposes development of agreements between the ARCBS and approved health providers (more specifically pathology laboratories) and, where appropriate, between jurisdictions and their approved health providers. The agreements aim to articulate the JBC’s safety and quality expectations of recipients of government funded blood and blood-related products, including supporting appropriate use of products by clinicians in hospitals.
A discussion paper and project plan were developed and discussed with ARCBS and South Australia, the jurisdiction chosen to pilot the agreements. Further work has been identified as necessary before agreements can be effectively implemented. This work includes research to gain an understanding of barriers to appropriate management and use of blood products within hospitals and pathology laboratories.
Product change proposals
Under the National Blood Agreement, interested parties can make proposals for changes to products or services on the National Products and Supply List. Schedule 4 of the National Blood Agreement provides for evidence-based evaluation, information and advice to support decisions on these changes.
In 2007–08, the NBA commissioned an academic health economics department to design purpose-built guidelines to evaluate national blood supply change proposals. It is expected that the guidelines will be finalised and implemented in 2008–09. A number of proposals have been submitted to the NBA and are currently being reviewed.
National Managed Fund
The National Managed Fund (NMF) was established by the Australian Health Ministers’ Advisory Council (AHMAC) to cover future liability claims made against the ARCBS in relation to the supply of blood and blood-related products within Australia. The NMF became effective from 1 July 2000, prior to the establishment of the NBA.
The National Indemnity Reference Group (NIRG) acts as a technical advisory subcommittee to the JBC on NMF-related matters and comprises Australian, state and territory government members. The NBA provides secretariat support to the NIRG, which met on 2 November 2007, and noted a number of significant achievements, including:
- endorsing of the actuarial and liability services reports by the management and advice support services provider, PricewaterhouseCoopers.
- establishing and implementing of the claims management system and processes, and data reporting and analysis by the claims management and advice services provider (Marsh Pty Ltd).
- implementing of comprehensive service level agreements and business plans with both service providers to the NMF.
- ensuring full awareness of the key aspects of ARCBS risk management arrangements in relation to blood-borne diseases.
The NIRG’s attention is now focused on further consideration of the scope to harmonise statutory defence under state and territory legislation.
< Previous | Contents | Next >
