Organisation at a glance
Our Vision
Saving and improving Australian lives through a world-class blood supply.
Our Role
The National Blood Authority (NBA) is a statutory agency within the Australian Government Health portfolio that manages and coordinates arrangements for the supply of blood and blood products and services on behalf of the Australian Government and state and territory governments.
The key role of the NBA is to:
- provide an adequate, safe, secure and affordable supply of blood products, blood related products and blood related services, and
- promote safe, high quality management and use of blood products, blood related products and blood related services in Australia.
The NBA:
- works with jurisdictions to determine the clinical requirements for blood and blood products and develop an annual supply plan and budget
- negotiates and manages national contracts with suppliers of blood and blood products to obtain the products needed
- assesses blood supply risk and develops commensurate contingency planning
- supports the work of the jurisdictions to improve the way blood products are used - including developing and facilitating strategies and programmes that will improve the safety, quality and effectiveness of blood usage, particularly in the areas of national standards, guidelines and data capture and analysis supports the work of the jurisdictions to improve the way blood products are used - including developing and facilitating strategies and programmes that will improve the safety, quality and effectiveness of blood usage, particularly in the areas of national standards, guidelines and data capture and analysis
- provides expert advice to support government policy development, including identification of emerging risks, developments, trends and new opportunities
- manages the evaluation of proposals for blood sector improvements, including proposals for new products, technologies and system changes
- provides secretariat support to the Jurisdictional Blood Committee (JBC).
Authority
The NBA was established by the National Blood Authority Act 2003 following the signing of the National Blood Agreement by all state and territory health ministers in November 2002. As a material statutory agency, the NBA has a range of corporate and compliance responsibilities under the National Blood Authority Act 2003, the Public Governance, Performance and Accountability Act 2013 (PGPA)1, and the Public Service Act 1999, along with a responsibility to meet ministerial, parliamentary and financial reporting requirements.
Responsible Ministers and Portfolio
The NBA exists within the portfolio responsibilities of the Minister for Health. The NBA General Manager is a statutory officer who reports to the Council of Australian Governments (COAG) Health Council and the Commonwealth Minister for Health.
Our Outcome
Access to a secure supply of safe and affordable blood products and coordination of best practice standards within agreed funding policies under the national blood arrangements.
Funding
Under the National Blood Agreement between the Australian Government and the states and territories, 63 per cent of NBA funding is provided by the Australian Government and the remaining 37 per cent is provided by the state and territory governments. The funding covers both the national blood supply and the operations of the NBA.
In the last ten years, governments have provided funding of $8,258.7 million for the supply of blood and blood products as detailed in Table 1.1. In 2013-14, the total amount provided was $1,095.9 million. Governments provided funding of $10 million in 2013-14 for the operation of the NBA.
TABLE 1.1 Government funding for the supply of blood and blood products, 2004-05 to 2013-14
| Year | Amount ($M) | Growth (%) |
|---|---|---|
| 2004-05 | 536.8 | 16.6 |
| 2005-06 | 577.4 | 7.6 |
| 2006-07 | 639.4 | 10.7 |
| 2007-08 | 719.5 | 12.5 |
| 2008-09 | 806.8 | 12.1 |
| 2009-10 | 878.8 | 8.9 |
| 2010-11 | 939.2 | 6.9 |
| 2011-12 | 1,015.6 | 8.1 |
| 2012-13 | 1,049.3 | 3.3 |
| 2013-14 | 1,095.9 | 4.4 |
| Total | 8,258.7 | 9.1 (average) |
1 The PGPA Act replaced the Financial Management and Accountability Act 1997 from 1 July 2014
Our Staff
As at 30 June 2014, the NBA had 46 ongoing and 10 non-ongoing staff. The organisational structure is shown at Figure 1.1.
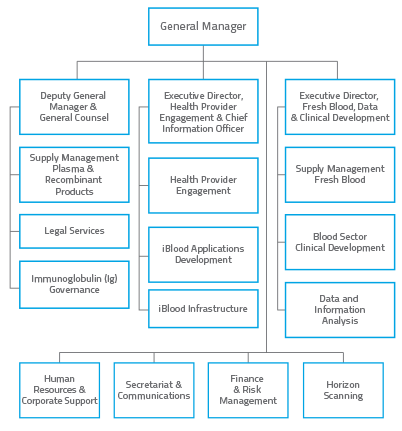
FIGURE 1.1 NBA Organisational Chart
Location
The NBA staff are located in Canberra at Level 2, 243 Northbourne Avenue, Lyneham ACT.
Key Events in NBA’s History
| 2003 | Established by the National Blood Authority Act 2003 following the signing of the National Blood Agreement by all state and territory health ministers in November 2002 |
| 2004 | Commencement of national supply arrangements for imported intravenous immunoglobulin (IVIg) to ensure sufficiency of supply in all jurisdictions |
| 2005 | Commencement of an adequate supply of recombinant Factors VIII and IX to fully meet demand |
| 2006 | NBA executed a Deed of Agreement with the Australian Red Cross Society for the provision of fresh blood products |
| NBA won the Prime Minister’s Silver Award for Excellence in Public Sector Management for procurement of recombinant (manufactured) products | |
| 2007 | First edition of Criteria for the clinical use of IVIg in Australia was approved |
| 2008 | Launch of the National Blood Supply Contingency Plan (NBSCP) to provide clear governance for managing blood shortages |
| Launch of the redeveloped Australian Bleeding Disorders Registry (ABDR) to better support planning and clinical management of people with bleeding disorders | |
| 2009 | Establishment of the Australian National Haemovigilance program to report on serious transfusion related adverse events |
| NBA was awarded with the Australian Government Comcover Award for Excellence in Risk Management for the NBSCP | |
| 2010 | New CSL Australian Fractionation Agreement came to effect |
| NBA won a United Nations Public Service Award in the Advancing Knowledge Management in Government category | |
| 2011 | National rollout of BloodNet, an online web based blood ordering system |
| Release of the first module (Critical Bleeding/Massive Transfusion) of the Patient Blood Management (PBM) Guidelines | |
| 2012 | Release of PBM Guidelines Module 2 Perioperative and Module 3 Medical |
| Second edition of IVIg Criteria in Australia was published | |
| 2013 | Release of PBM Guidelines Module 4 Critical Care |
| Inaugural National Blood Symposiums conducted in Sydney, Melbourne and Adelaide | |
| 2014 | National rollout of MyABDR |
| Inaugural PBM Conference held in Perth | |
| Immunoglobulin governance programme and National Immunoglobulin Governance Advisory Committee established |
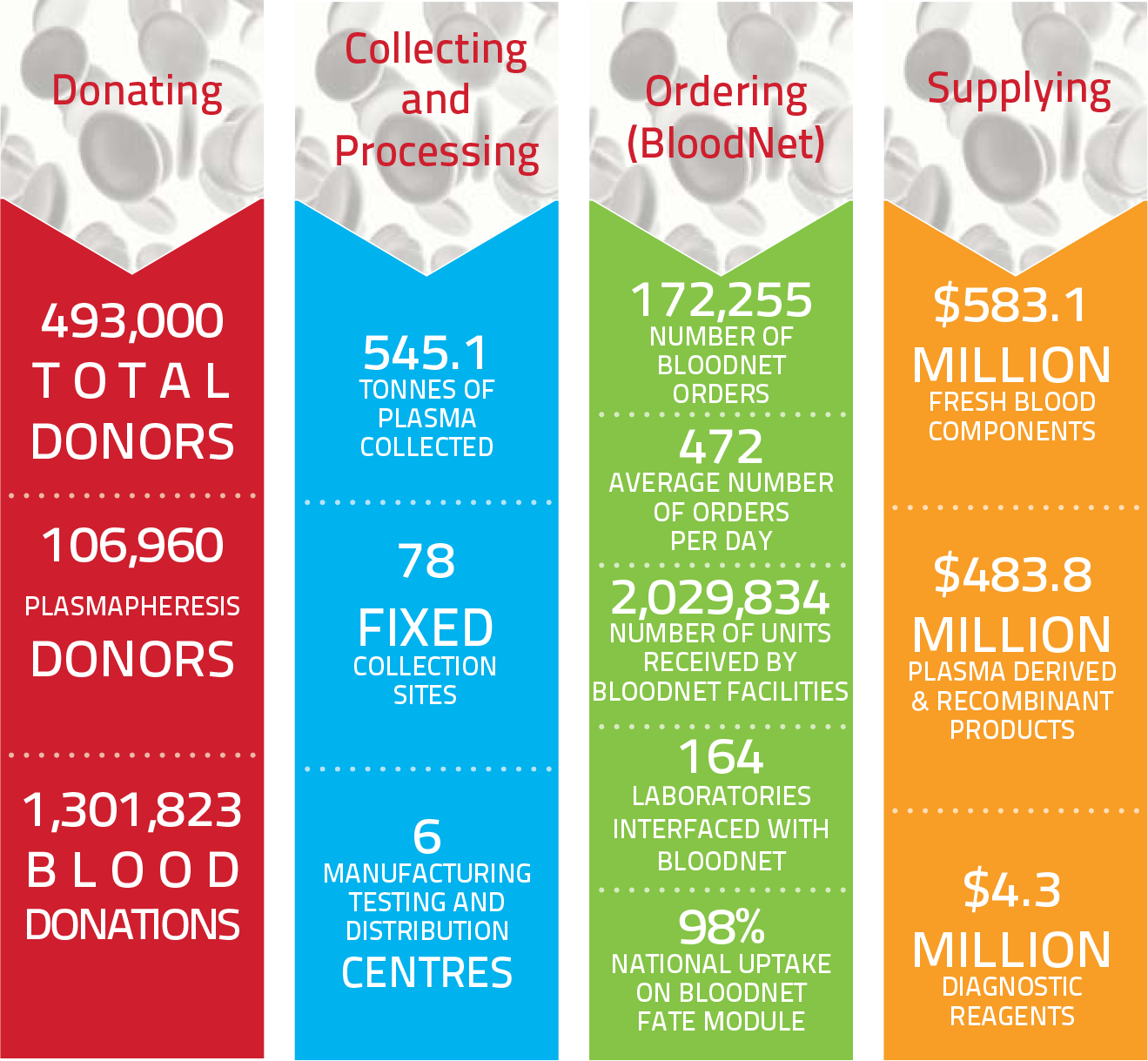
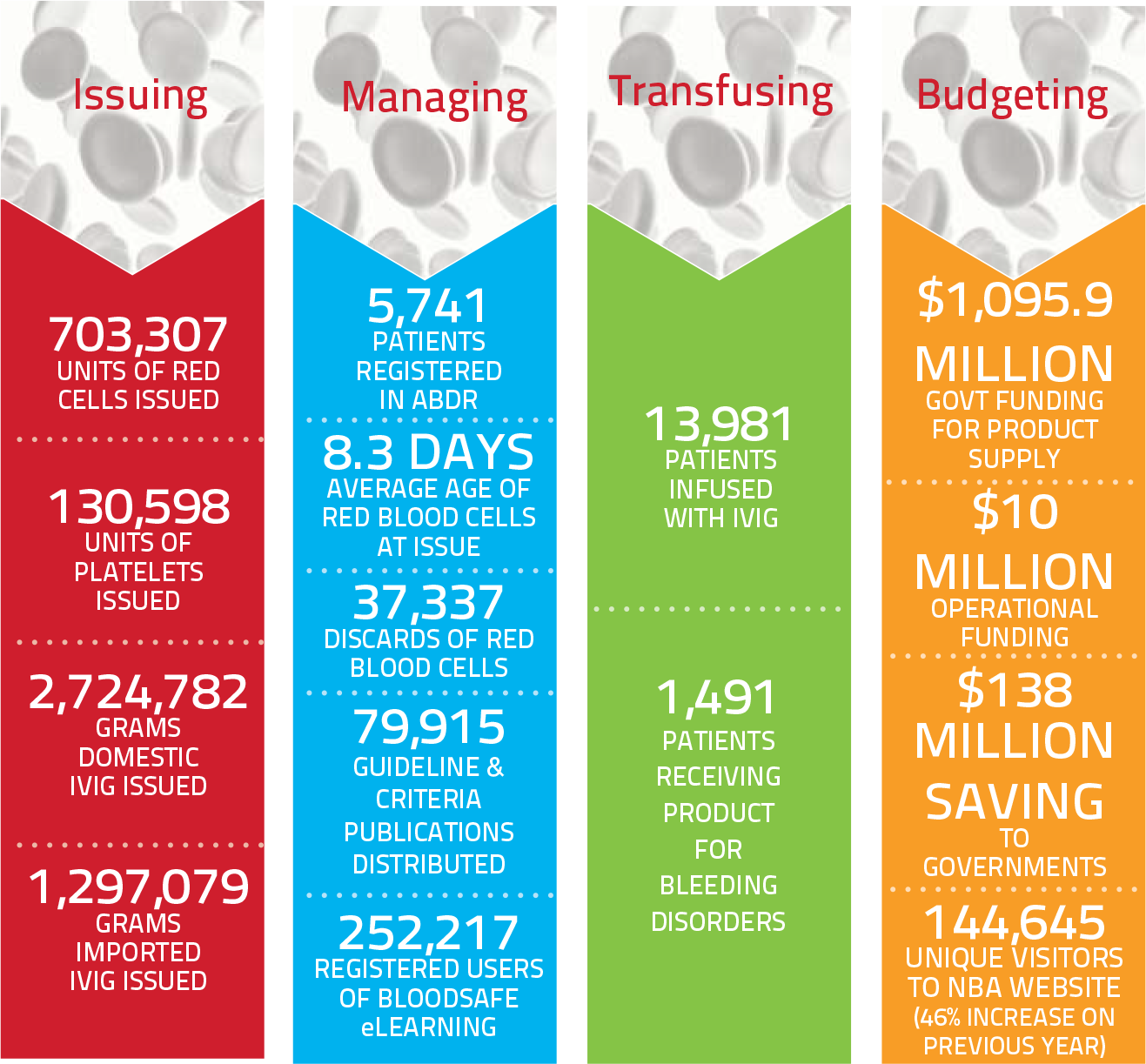
Year at a Glance: Snapshot of the blood sector in 2013-14
Key Achievements
DELIVERY OF UNINTERRUPTED SUPPLY TO MEET CLINICAL DEMAND AT A RECORD SAVING OF $138 MILLION
NATIONAL ROLLOUT OF MYABDR
NATIONAL TENDER FOR RECOMBINANT PRODUCTS ACHIEVES AN ANNUAL SAVING OF $50 MILLION PER ANNUM
AN ARRAY OF BEST PRACTICE TOOLS AND CASE STUDIES SUPPORTING THE IMPLEMENTATION OF PATIENT BLOOD MANAGEMENT AND IMPROVED INVENTORY MANAGEMENT DEVELOPED AND PUBLISHED
Key Achievements
BLOODNET INTERFACES WITH HOSPITAL LABORATORY INFORMATION SYSTEMS ESTABLISHED TO COVER 20 PER CENT OF ISSUED VOLUME OF BLOOD AND BLOOD PRODUCTS
COMPREHENSIVE NATIONAL REVIEW OF RISK IN THE BLOOD SECTOR COMPLETED
THE FIRST NEW BLOOD PRODUCT TO BE ADDED TO THE NATIONAL SUPPLY LIST, SUBCUTANEOUS IMMUNOGLOBULIN (SCIG), BECOMES AVAILABLE
