You are here
Objective 2. Improve risk management and blood sector performance
In 2017-18, the NBA achieved a range of objectives to improve blood sector performance and risk management, particularly in the areas of Ig governance, evaluation of new products, ICT developments, data availability and analysis and risk and knowledge management.
Ig Governance
The NBA Ig Governance Program continued its work throughout 2017-18 to improve the Governance and management of publicly funded Ig. This program aims to ensure:
- Ig product use and management reflects appropriate clinical practice and represents efficient, effective and ethical expenditure of government funds, in accordance with relevant national safety and quality standards for health care
- access to Ig products is consistent with the Criteria for access determined by governments
- improved capture of information on the need for, use of, and outcomes of treatment (including adverse events) with Ig products to inform future changes to the Criteria.
During the 2017-18 period, the program focused on the four key activities listed below:
- Development and commencement of an implementation strategy to launch BloodSTAR (Blood System for Tracking Authorisations and Reviews) in New South Wales
- Development of a performance improvement strategy focused on enhanced management and use of Ig
- Finalisation and approval Version 3 of the Criteria and commencement of a transition strategy including via BloodSTAR
- Engagement with the program's network of committees to deliver the activities listed above, and plan for the next three years.
Launch of BloodSTAR in New South Wales
The BloodSTAR system was developed by the NBA on behalf of all Australian governments to support health providers in managing their Ig governance obligations as set out in the National Policy: Access to Government-Funded Ig Products in Australia. The system standardises and manages access to the supply of immunoglobulin products by enabling authorisation requests to be submitted electronically and work-flowed to an authoriser for assessment and approval.
In 2017-18, the NBA worked with the New South Wales Ministry of Health to develop and finalise an implementation plan to launch BloodSTAR in its jurisdiction in late 2018. Currently there are approximately 4,000 patients receiving Ig treatment in New South Wales.
Implementation activities will involve the migration of data for NSW patients currently authorised to receive Ig into BloodSTAR as well as providing training and support for BloodSTAR users in the lead up to the launch.
The launch of BloodSTAR in New South Wales will complete the national rollout of the system enabling collection of improved national data and enhancement of the NBA's ability to further develop the Criteria and provide an improved evidence base for practice improvement and research into the future.
Performance Improvement Strategy
In 2017-18 the NBA commenced work on the development of a performance improvement strategy focused on enhanced management and use of Ig. The strategy will aim to monitor performance and promote continuous improvement across the Ig governance system.
Activities identified in the strategy will be focused, cohesive and will relate to the objectives of the Ig Governance Program. They will also align with five key performance areas:
- provision of Ig reflects appropriate clinical practice
- uniform compliance with the National Policy
- local Ig governance arrangements are robust and align with relevant standards, guidelines and legislative requirements
- service delivery is efficient and effective
- data collection supports future work.
The strategy will identify education, training and support activities to support health professions that are involved in the management and use of Ig. It will also identify communication activities and a strategy to strengthen current relationships and build new relationships with stakeholders both in Australia and abroad. Data collection, analysis and reporting activities will also be featured and will provide assurance that the Ig Governance Program is successfully directing government-funded Ig products to patients that benefit and that the program represents efficient, effective and ethical expenditure of government funds. Knowledge development activities will support further policy development in the future, promoting sustainability and enabling the Ig Governance Program to be responsive to change including in response to new advances in research.
The Ig Governance Program's network of committees, and more specifically, the Specialist Working Groups have made major contributions to the development of the strategy which will continue in 2018-19.
Revision of the Criteria for the Clinical Use of Immunoglobulin in Australia
The Criteria for the Clinical Use of Intravenous Immunoglobulin in Australia (the Criteria) ensures that Ig funded under the national blood arrangements is accessed consistently across Australia for the treatment of patients whose health is likely to be improved with Ig therapy.
The Criteria is based on evidence identified through systematic reviews of the literature and the opinions of clinical experts. Version 1 of the Criteria was first published in 2007 and updated to Version 2 of the Criteria in 2012. A complete revision was undertaken in 2015 and approved in 2017. The NBA will be releasing Version 3 of the Criteria in BloodSTAR on 22 October 2018.
Developed by Specialist Working Groups (SWG) including practicing neurology, immunology, haematology and transplantation specialists and in collaboration with relevant clinical colleges and societies, Version 3 of the Criteria aims to more clearly articulate and standardise the diagnostic, qualifying and review criteria, initial and continuing authorisation periods, dosing controls and supporting evidence for access to publicly funded Ig.
The revision process involved:
- review of all qualifying, review and dose criteria for conditions in Version 2 of the Criteria
- review of evidence items and system controls
- over 55 meetings of the SWG where the available evidence was reviewed and revisions to the Criteria were formulated
- two public consultation rounds to allow the broader community to consider proposed revisions and provide feedback
- consideration and endorsement of the proposed changes by the National Immunoglobulin Governance Advisory Committee (NIGAC) and the Jurisdictional Blood Committee (JBC)
- comprehensive quality assurance analysis of all endorsed changes to ensure accurate translation via the BloodSTAR system
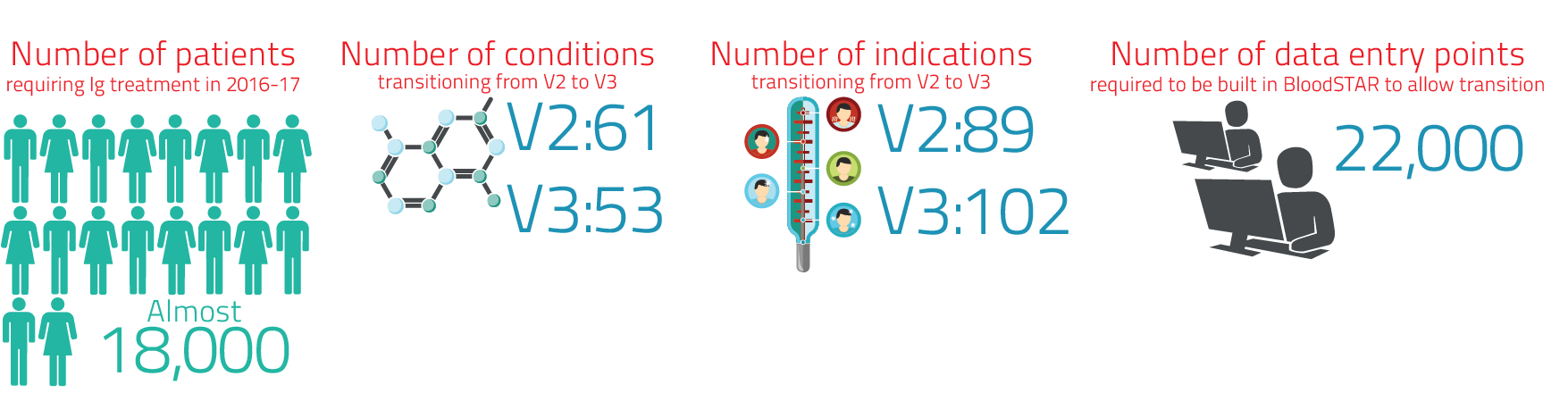
- the development of approximately 22,000 descriptor and system controls to support BloodSTAR functionality
- an upgrade of BloodSTAR to support Version 3 of the Criteria
- extensive planning to support the transition of over 18,000 patient records from Version 2 of the Criteria to Version 3 of the Criteria.
The revised Criteria
Developed through systematic reviews of the literature and the opinions of clinical experts, Version 3 of the Criteria ensures that Ig funded under the national blood arrangements is accessed consistently across Australia for the treatment of patients whose health is likely to be improved with Ig therapy.
Clinicians and transfusion medicine professionals can use the Criteria to identify the conditions and circumstances for which immunoglobulin products are available for use under the national blood arrangements.
Version 1 of the Criteria was first published in 2007 and updated to Version 2 of the Criteria in 2012. A complete revision commenced in 2015-16 and continued in 2016-17 (Version 3). This revision aimed to more clearly articulate and standardise the diagnostic, qualifying and review criteria, initial and continuing authorisation periods, dosing controls and ensure the submission of supporting evidence for access to funded Ig. This strengthening of the Criteria will help ensure:
- prescription of Ig is consistent with current evidence and expert opinion
- sustainability of Ig supply by ensuring continued availability for patients who derive genuine health benefits
- treatment decisions involve careful consideration of alternative therapies, prescribing the lowest effective dose, and appropriate weaning from Ig therapy
- ethical expenditure of government funds in accordance with relevant national safety and quality standards for health care.
In 2017-18 the NBA worked with Specialist Working Groups to finalise proposed changes to the Criteria under the auspices of the National Immunoglobulin Governance Advisory Committee (NIGAC) prior to consideration by the JBC. Following endorsement, a transition strategy commenced to support the release Version 3 of the Criteria including via BloodSTAR.
The process required the review of all qualifying, review and dose criteria, along with evidence items and system controls. To ensure accurate translation via BloodSTAR, the definition and description of each evidence item and its associated system controls were subject to a comprehensive quality assurance analysis. Approximately 22,000 descriptor and system controls needed to be defined for the revised Criteria to be translated in BloodSTAR. The description of such fields determines:
- what the requestor sees
- what the requestor is asked to provide
- whether the requirement is mandatory or not
- instructions that will be provided to assessors.
Version 2 of the Criteria comprises sixty one conditions and eighty nine indications which have been mapped to the fifty three conditions and 102 indications in Version 3 of the Criteria as part of the implementation strategy. Existing patient authorisation records require transition to the new version and a transition strategy was developed depending on the current indication for Ig use and whether or not it allowed ongoing treatment in either version. Potentially over 18,000 patient records will require transition to the new version. To accommodate Version 3 of the Criteria, a major upgrade to functionality of BloodSTAR also commenced in 2017-18.
Version 3 of the Criteria is expected to be released in October 2018 and will be relevant for compliance with the Blood Standard of the National Safety and Quality Health Service Standards (Standard 7 - Blood Management).
Network of Committees
In 2017-18 the NBA continued to work with the Ig Governance Program's network of committees to deliver program activities including the projects outlined above.
The program's principal committee, NIGAC, met four times during the year to provide advice to the NBA on particular aspects of the Ig Governance Program and to oversee the work of Specialist Working Groups. Key projects that involved NIGAC and Specialist Working Groups specifically in 2017-18 include the review of Version 3 of the Criteria including the transition planning for Version 3 of the Criteria and the development of the Performance Improvement Strategy.
The Haematology, Immunology and Neurology Specialist Working Groups also participated in planning days to inform work plans and set priorities for the next 3 years. Work plans were endorsed by NIGAC and comprise activities that will support the NBA to ensure Ig governance goals continue to be met. A work plan for the Transplantation Specialist Working Group will be developed in 2018-19.
Throughout the year, the NBA engaged with jurisdictional health departments and Ig interest groups established by State and Territory health departments to support Ig governance arrangements. Engagement with these groups ensures transparency between the program and jurisdictions, and creates channels to communicate issues, provide feedback, and facilitate understanding of issues.
In 2017-18, an additional group, the National Ig Interest Group, was established as part of the network of committees. This group connects the program to a broader network of stakeholders such as clinicians, dispensers, nurses, facility administrators and consumers.
Evaluation of new products
A working group established by the JBC, including NBA and jurisdictional representatives, progressed work on the requirements and processes for evaluations to be undertaken under Schedule 4 of the National Blood Agreement.
Following advice from the NBA, the JBC endorsed proposals for the following products to be referred to the Medical Services Advisory Committee (MSAC) for consideration of a health technology assessment and possible inclusion on the National Supply List:
- Extended half-life clotting factor concentrates for treatment of haemophilia A and B
- Emicizumab for routine prophylaxis in patients with congenital haemophilia A with factor VIII inhibitors
- Purified human alpha1-proteinase inhibitor for the treatment of alpha1-proteinase inhibitor deficiency, leading to chronic obstructive pulmonary disease.
The above assessments are well underway and the outcomes of the MSAC review process are expected to be considered by the JBC during 2018-19.
Data developments
In 2017-18, the NBA continued to build its data capture and analysis capabilities across all aspects of the supply chain. This area of activity is a key strategy to improve the overall efficiency and sustainability of the sector by providing a measurement for improvement.
A significant amount of data and information exists within the blood sector, however, the extent to which this data is currently available to the parties that need it, the quality of the data, and the capacity of the systems that hold it, varies widely. During 2017-18, the NBA progressed the following activities:
- continued to develop the list of system reports to be provided to stakeholders and developed specifications to assist in their development
- refined and implemented monthly and quarterly issue reports to be provided to stakeholders
- discard data:
- collected, analysed and distributed discard data from the BloodNet Fate Module to support the establishment of revised targets for discard rates under the National Blood and Blood Product Wastage Reduction Strategy 2013-2017
- developed and distributed further BloodNet discard reports on red blood cell ABO groups and reporting by public and private health providers.
- Haemovigilance
- developed the annual National Haemovigilance Report 2017 based on data for 2014-15 collected by States and Territories. Donor vigilance data was collected from the Blood Service
- developed the annual National Haemovigilance Report 2018 based on data for 2015-16 collected by States and Territories. Donor vigilance data was collected from the Blood Service
- refined the work plan to support implementation of the Strategic Framework for the National Haemovigilance Program approved in 2014-15
- established working groups to continue to develop haemovigilance tools
- reviewed the Australian Haemovigilance Minimum Data Set for haemovigilance reporting
- Developed and implemented the National Blood Product Management Improvement Strategy 2018-22 (Improvement Strategy)
- Australian Bleeding Disorders Registry (ABDR):
- developed the ABDR Annual Report for 2016-17
- continued to develop the set of data standards as part of the data integrity process for the ABDR for review by AHCDO Executive and the Data Managers
- provided to AHCDO the 2016-17 ABDR Benchmarking Report
- the NBA signed Information Framework Agreements with South Australia in 2014-15, with Northern Territory, Tasmania and Western Australia in 2015-16 and New South Wales in 2016-17 and Australian Capital Territory in 2017-18. These agreements are required as part of the National Blood Sector Data and Information Governance Framework:
- developed the Ig Annual Report for 2016-17 from BloodSTAR and STARS
- responded to 110 data requests from internal and external stakeholders.
Risk management
Risk management remains a core focus within the NBA, underpinning the ongoing commitment to managing the national blood supply.
Recent attention to risk has seen a review of the Risk Management Policy and Framework which was endorsed in August 2017 by the Chief Executive. Annual validation of the NBA strategic risks also shows a need to be vigilant in not only the potential interruption to supply and services in the blood sector but also in consideration of enhanced products and services as well as attention to major systems and infrastructure.
National Blood Supply Contingency Plan (NBSCP)
The NBSCP is the specific risk plan to address potential interruptions for blood and blood products supply within Australia.
Ongoing enhancements and planning for the NBSCP include:
- in March 2018 the JBC endorsed a revised version of the NBSCP with Attachments A-E covering product specific contingency arrangements
- development of a simulation exercise to test the plan or elements of the plan, to commence in 2018-19
- broadening contingency management arrangements for events not presently encompassed within the current plan such as non-product based NBSCP activations.
Business Contingency Plan (BCP)
The NBA has a robust BCP to address any potential or significant interruption to normal business operations.
In 2017-18 the NBA:
- conducted a business impact analysis of the NBA business functions as the basis of recovery planning
- expanded the suite of core business processes required for immediate restoration through any interruption
- validated the core business resources required to be 'on hand' through different re-establishment of services processes.
Supply Risk Mitigation for Plasma Derived and Recombinant Products
Specific risk management strategies have been developed and implemented for individual plasma and recombinant products. The plasma and recombinant supply risk assessment is updated annually. The NBA completed the update for 2017-18. The update included the validation of existing stock and contractual supply reserves.
Information Communication Technology (ICT)
The NBA Information and Technology Services Group (ITSG) continue to provide technology solutions and high quality support for NBA staff and deliver approved JBC strategies and projects. ICT projects and systems are a key enabler of both data collection/analysis and business process reform across the sector. These enabling initiatives continue to deliver the following high level outcomes:
- policy compliance
- infrastructure updates
- platform enhancements
- reporting capability
- ABDR and MyABDR enhancements
- BloodNet enhancements
- BloodSTAR enhancements
- BloodNet–LIS interface.
All BloodNet–LIS interfaces currently in place are responsible for processing 37 per cent of total national issues of fresh blood products.
ICT Committee
During 2017-18 the ICT Committee met seven times. The NBA's Senior Management Group comprised membership of the Committee. Representatives from relevant sections of the NBA were also in attendance.
The Committee oversees the NBA technology, information and data management strategic programs and during 2017-18 focused on the delivery of projects under a suite of inter-related ICT development work described as the Crimson Program and improvements to data and information management capabilities such as:
- commenced drafting the Blood Sector Data and Systems roadmap to guide activities after the Crimson Program 2016-2019
- oversight of delivery for 1 July 2018 of BloodNet 5
- a proof of concept with Queensland Health to explore the benefits of interfacing BloodSTAR with their BloodNet/LIS – an outstanding success saving facilities significant time and double entry of data
- continued improvements to BloodSTAR version 2 and the development of the soon to be released BloodSTAR version 3
- continued improvements to ABDR and MyABDR
- nationwide training for blood sector systems users
- ongoing support, security patching and technical issue resolution for all NBA blood sector systems.
BloodNet redevelopment
Background
BloodNet is Australia's online blood ordering and inventory management system. BloodNet provides the ability for Australian hospitals and laboratories to order blood and blood products and is supported by a twenty four hour service to ensure that essential, lifesaving products get to where they are needed. Approximately 28,000 litres of blood are ordered and tracked each month.
BloodNet
The new version of BloodNet, BloodNet 5, will be released to users on 1 July 2018.
The redevelopment of BloodNet incorporated major interface design changes to enhance usability, streamline functions and deliver a modern look and feel.
NBA ICT staff travelled across the country and conducted training sessions in May and June 2018 to give users the opportunity to familiarise themselves with the new system.
Face to face training to nominated locations in every state and territory and WebEx (web conference) training sessions were conducted.
BloodNet 5 – user involvement
Throughout the project, the NBA regularly consulted with users via the BloodNet User Reference Group (BURG) to ensure the design and development of the system would meet the needs of our users. Sessions via telephone, video-conferencing and in the NBA's Canberra office allowed the chance to co-design and present the developing system and the NBA is grateful for the feedback of this group.
Many changes were made along the way to ensure the system was user friendly and delivers what users within the sector need.
User testing of BloodNet 5 occurred in May 2018 with over twenty users from around Australia participating in two days of system testing.
Some of the comments on the new BloodNet 5 include:
'the layout is clear and easy to use, I don't think anyone who is familiar with the previous BloodNet will struggle to fill out orders or receipt products'
'system is really easy to use - can see most of the staff picking it up without any problem'
'I have worked through the suggested scenarios and found the system easy to use and very intuitive. I made errors and the prompts let me know what was wrong'.
Recording Ig dispense episodes
BloodNet interacts with BloodSTAR to help with ordering and dispensing of correct products to authorised patients. Dispensers use the Authorisation tab in BloodNet which contains all authorised patient information linked to their facility.
To meet obligations set out in the National Policy: Access to Government-Funded Immunoglobulin Products in Australia all Ig dispense episodes must be recorded in BloodNet. Dispensers are responsible for ensuring that Ig is dispensed in accordance with the authorisation. The information in the BloodNet Authorisation tab will assist the dispenser to ensure that only authorised patients are dispensed Ig products, and that they dispense the correct product and dose, at the correct intervals.
Recognition
The Digital Transformation Agency acknowledged the NBA's work with BloodNet 5 and the NBA also received an award for the digital transformation of BloodNet 5.

Australian Bleeding Disorders Registry (ABDR)
The ABDR and associated patient portal (MyABDR) is a clinical tool used on a daily basis by clinicians in all Australian haemophilia treatment centres to assist in the management and treatment of people with bleeding disorders. The NBA delivered a number of updates and improvements in 2017-18 to enhance the functionality and user experience with both ABDR and MyABDR. These changes included:
- updates to the security requirements in order to protect sensitive information on MyABDR
- assorted user requested functionality updates.
BloodNet
The BloodNet redevelopment project concluded in 2017-18 with implementation of the new system scheduled for 1 July 2018. Throughout the project the NBA regularly consulted with users to ensure the new system was designed and developed around the needs of the user. The BloodNet User Reference Group (BURG) was involved at regular intervals and helped to test the system prior to release. We are grateful for their feedback which has resulted in a successful system delivery. Program outcomes for 2017-18 were:
- In August 2017, BloodNet 5 was the first to pass the government's Digital Service Standard alpha assessment and in January 2018, BloodNet 5 was the first system development to achieve a beta assessment without requiring further support or guidance from the Digital Transformation Agency, highlighting the successful approach in ensuring the system was designed around end user needs
- The NBA received the Open Gov Recognition of Excellence Award 2018 for the digital transformation platform – BloodNet 5
- The system has received very positive reviews and feedback from users and stakeholders.

Image: BloodNet User Reference Group
'Recognition of Excellence 2018' digital transformation platform – BloodNet 5 OpenGov Award

L to R: Mr Simon Spencer, NBA's CIO, receiving the award from Mr Mohit Sagar, Editor in Chief and MD of Open Gov.
OpenGov recognised seven government agencies in Canberra, Australia for using technology to improve citizen engagement and public service delivery.
On 5 June 2018, OpenGov recognised the NBA for the transformation of its digital platform for ordering and receipting blood and blood products. The NBA's Chief Information Officer accepted the award from OpenGov.
OpenGov's comments on the NBA award were as follows:
'The NBA manages the ordering of blood and blood products around Australia. It operates a twenty four hour service to ensure that essential life-saving blood and blood products get to where they're needed. Approximately 28,000 litres of blood are ordered and tracked through its platform each month.
An online system for ordering and tracking blood products called ORBS (Ordering, Receipting Blood System) was developed by the Queensland Department of Health first developed in 2009. This was proposed to the NBA as a national system in 2010. A bespoke application called BloodNet was developed.
The BloodNet version five was developed to not only upgrade the technology and platforms, but also create a new look user interface. The NBA relied on the DTA's Digital Service Standard to ensure its services are simple, clear, and in line with user's needs. During the development process, it brought a multi-disciplinary team in-house and upskilled internal staff. The team adapted to agile principles, tools and techniques, and held fortnightly sprints, daily stand-ups, regular walk-throughs and responded to feedback. A total of 187 hours of user research was conducted during the discovery and alpha stages, which included visiting 138 people across thirty nine hospitals/laboratories in nine different cities.
Through this process, the team designed a simpler platform that makes it faster and easier for hospitals to order and receive life-saving blood products. It became the first agency – that is required to use the Digital Service Standard – to pass its alpha assessment independently. The product is in Beta and the team is working on ensuring the system is built for longevity and ease of maintenance; setting up release process and transition plans for users; identifying appropriate metrics and KPIs for the performance dashboard; and continuing user research and testing to make sure we are on the right track.'
2017-18 Sector monitoring
In 2017-18, the NBA continued its horizon scanning of international experience that may influence the management of blood and blood products in Australia. This monitoring activity informs the provision of current and proactive analysis to governments to enable the NBA to fulfil its functions under the National Blood Agreement.
Our focus in 2017-18 was:
- new product developments and applications
- global regulatory and blood practice trends
- scientific and clinical research with implications for supply or demand in the sector
- business events that may have an impact on global supply, demand and pricing, such as changes in company
- structure, financial outlook, production capacity, organisation, ownership, and marketing and contractual arrangements
- diseases or pandemics that may affect supply or risks to product safety
- developments in testing methods, vaccines and disease control strategies that could potentially mitigate risks to supply
- any other emerging risks that could potentially put financial or other pressures of any kind on the Australian sector.
The NBA regularly posts to its website a selection of items from this horizon scanning process, illustrating the wide range of factors which may influence industry operations and patient outcomes. This information is available www.blood.gov.au/monitoring-international-trends-blood-sector
During 2017-18, key developments included:
- continuing development of extended half-life clotting factors for haemophilia
- approval in the US and Japan of a humanised bispecific monoclonal antibody engineered to mimic the function of factor VIII (and simultaneously bind factors IXa and X) for use in patients with factor VIII inhibitors
- some encouraging results in research on gene therapies for haemophilia, sickle-cell disease and beta thalassemia, with some concerns remaining
- finding whole genome sequencing improves matching in blood transfusions
- confirming that repeated blood draws can lead to hospital-acquired anaemia
- development of cell-like nanorobots powered by ultrasound to clear bacteria and bacterial toxins from blood
- a global trial of heat-stable Carbetocin to prevent post-partum bleeding
- trialling of a plasma-derived product to reduce recurrent cardiovascular events following heart attacks
- expansion of approved uses for immunoglobulin
- more safety and efficacy data on reversal agents for novel anti-coagulants
- increased interest in non-invasive foetal RhD genotyping
- technological advances in freezing and thawing blood
- progress in the Takeda Pharmaceutical Company bid to acquire Shire Plc
- expansion of plasma product manufacturing facilities around the world.
Perth Processing Centre Refurbished
The formal launch of the refurbished Australian Red Cross Blood Service Processing Centre in Perth was held on 14 December 2017. A plaque to commemorate the event was unveiled by Ms Shelly Park, Chief Executive of the Australian Red Cross Blood Service. The building was purpose-built for the Blood Service in 1958 and it has been in continuous operation since then. It is a critical part of the Blood Service's infrastructure and is of significant importance to the WA health system. The facility is responsible for approximately eight per cent of the national blood supply. The refurbishment, funded by Australian governments, is part of a capital works program that commenced in 2013 at a cost of $43.5 million. The new state-of-the-art facility enables manufacturing to be configured for maximum work flow and allows for more efficient blood processing.

L to R: Mr Brett King, Manager of the Processing Facility, Blood Service Perth; Prof Lyn Beazley AO, NBA Board; Mr Terry Healy MLA, Member for Southern River WA; and Mr John Cahill, Chief Executive of the NBA, at the opening of the refurbished Perth Processing Centre.

L to R: Professor David Forbes, JBC, Mr John Cahill Chief Executive NBA, Ms Shelly Park Chief Executive of the Australian Red Cross Blood Service and a young recipient of life saving red cell transfusions, Miss Ellie Chin with her family.
