You are here
Organisation at a Glance
Our Vision
Saving and improving Australian lives through a world-class blood supply.
Our role
The National Blood Authority (NBA) is a statutory agency within the Australian government health portfolio that manages and coordinates arrangements for the supply of blood and blood products and services on behalf of all Australian governments.
The primary objectives under the National Blood Agreement are to:
- provide an adequate, safe, secure and affordable supply of blood products, blood related products and blood related services
- promote safe, high quality management and use of blood products, blood related products and blood related services in Australia.
The NBA
- works with all Australian Governments to determine the clinical requirements for blood and blood products and develop an annual supply plan and budget
- negotiates and manages national contracts with suppliers of blood and blood products to obtain the products needed
- assesses blood supply risk and develops commensurate contingency planning
- supports the work of all Australian governments to improve the way blood products are governed, managed and used – including developing and facilitating strategies and programs that will improve the safety, quality and effectiveness of blood usage, particularly in the areas of national standards, criteria, guidelines and data capture and analysis
- works collaboratively with key stakeholders to provide expert advice to support government policy development, including identification of emerging risks, developments, trends and new opportunities
- manages the evaluation of proposals for blood sector improvements, including proposals for new products, technologies and system changes
- provides secretariat support to the Jurisdictional Blood Committee (JBC).
Authority
The NBA was established by the National Blood Authority Act 2003 (NBA Act) following the signing of the National Blood Agreement by all state and territory Health Ministers in November 2002. As a material statutory agency, the NBA has a range of corporate and compliance responsibilities under the NBA Act, the Public Governance, Performance and Accountability Act 2013 (PGPA Act), and the Public Service Act 1999, along with a responsibility to meet ministerial, parliamentary and financial reporting requirements.
Accountable Authority
Details of the NBA's Accountable Authority during the current report period (2018-19) appear in Table 1.2 below.
TABLE 1.2 Details of Accountable Authority during the current report period (2018-19)
| Name | Position title/ position held |
Date of commencement | Date of cessation |
| Mr John Cahill | Chief Executive | October 2016 | n/a |
Responsible Ministers and Portfolio
The NBA exists within the portfolio responsibilities of the Minister for Health. The NBA General Manager is the Chief Executive of the NBA and is a statutory officer responsible to the Commonwealth Minister for Health and the Council of Australian Governments (COAG) Health Council.
Our Outcome
Access to a secure supply of safe and affordable blood products, including through national supply arrangements and coordination of best practice standards within agreed funding policies under the national blood arrangements.
Funding
Under the National Blood Agreement between the Australian Government and the states and territories, 63 per cent of NBA funding is provided by the Australian Government and the remaining 37 per cent is provided by the state and territory governments. The funding covers both the national blood supply and the operations of the NBA.
In the last ten years, governments have provided funding of $10,345.6 million for the supply of blood and blood products as summarised in Table 1.3. In 2018-19, the total amount provided was $1,203.6 million. Governments provided funding of $9.9 million in 2018-19, for the operation of the NBA.
TABLE 1.3 Government funding for the supply of blood and blood products, 2009-10 to 2018-19
| Year
|
Amount ($M)
|
Growth (%)
|
|---|---|---|
| 2009-10 | 878.8 | 8.9 |
| 2010-11 | 939.2 | 6.9 |
| 2011-12 | 1,015.6 | 8.1 |
| 2012-13 | 1,049.3 | 3.3 |
| 2013-14 | 1,095.9 | 4.4 |
| 2014-15 | 922.7 | -15.8 |
| 2015-16 | 1,040.9 | 12.8 |
| 2016-17 | 1,046.3 | 0.5 |
| 2017-18 | 1,153.3 | 10.2 |
| 2018-19 | 1,203.6 | 4.4 |
| Total | 10,345.6 | 3.9 (average) |
Note: Figures balance to the Audited Financial Statements
Our Staff
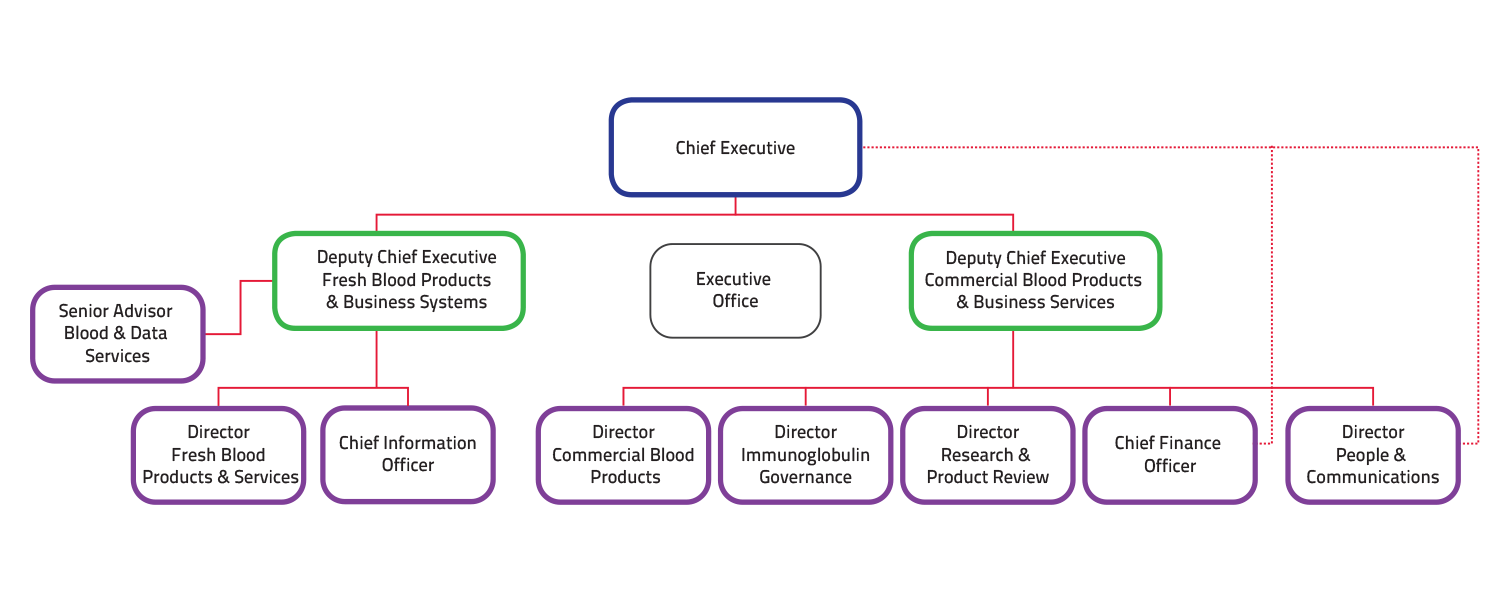
As at 30 June 2019, the NBA had an average staffing level of 55.62 staff. At this date, it also employed 23 contract staff. The organisational structure at 30 June 2019 is shown at Figure 1.1.
FIGURE 1.1 NBA Organisation as at 30 June 2019
Location
The NBA is located in Canberra at 243 Northbourne Avenue, Lyneham ACT.
Key Events in the NBA's History by Financial Year
| 2003 |
|
| 2004 |
|
| 2005 |
|
| 2006 |
|
| 2007 |
|
| 2008 |
|
| 2009 |
|
| 2010 |
|
| 2011 |
|
| 2012 |
|
| 2013 |
|
| 2014 |
|
| 2015 |
|
| 2016 |
|
| 2017 |
|
| 2018 |
|
| 2019 |
|
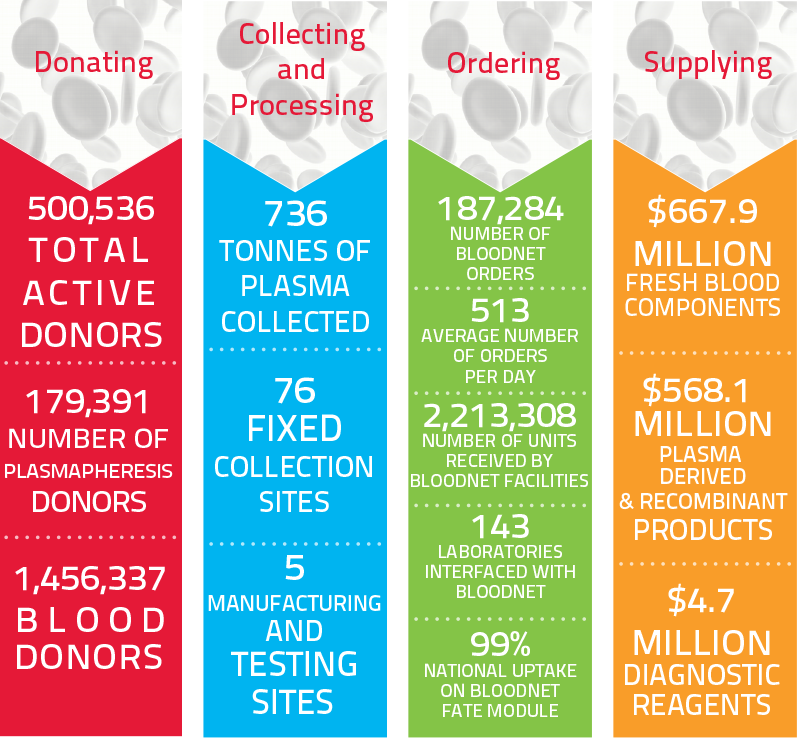
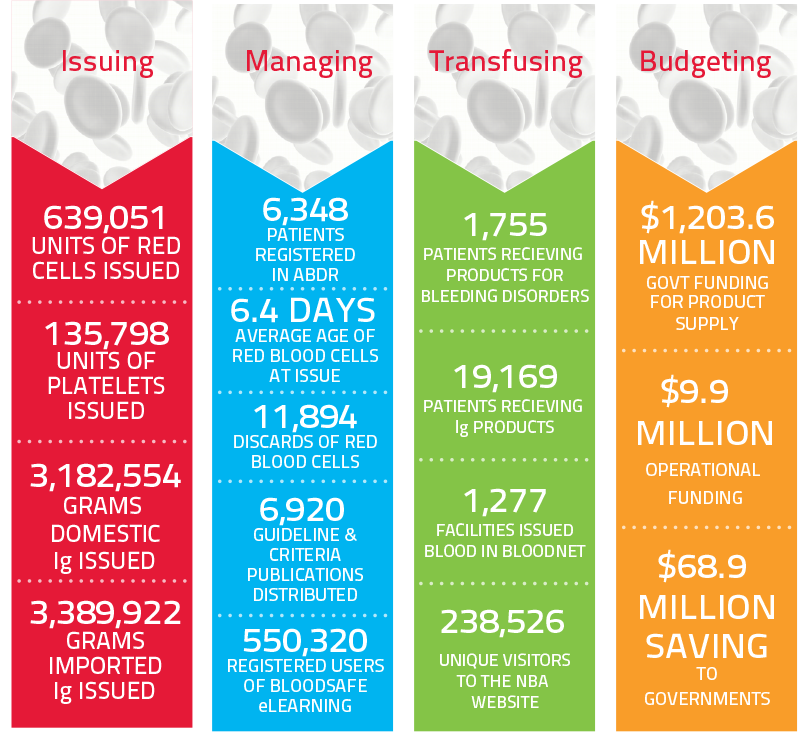
Year at a Glance: Snapshot of the blood sector in 2018-19
KEY ACHIEVEMENTS
DELIVERY OF UNINTERRUPTED SUPPLY TO MEET CLINICAL DEMAND AT A SIGNIFICANT SAVING OF $68.9 MILLION
BLOODNET REDEVELOPMENT RELEASED IN ALL JURISDICTIONS
BLOODSTAR FULLY IMPLEMENTED AS A NATIONAL SYSTEM
VERSION 3 OF THE CRITERIA FOR IMMUNOGLOBULIN RELEASED IN BLOODSTAR IN OCTOBER 2018
KEY ACHIEVEMENTS
A REDUCTION IN RED BLOOD CELL WASTAGE FROM 2.2% to 1.9%
NATIONAL BLOOD SECTOR RESEARCH AND DEVELOPMENT PROGRAM FUNDING FOR ROUND FOUR COMMENCED
CONSULTATION FOR POTENTIAL FUTURE ARRANGEMENTS FOR IMPORTED PLASMA AND RECOMBINANT PRODUCTS COMPLETED
CONTINUED LIMITED INTERIM ARRANGEMENTS FOR EXTENDED HALF-LIFE CLOTTING FACTOR PRODUCTS
