Available Australian haemovigilance data for 2011-12 and 2012-13
The NBA established a National Haemovigilance Program and the HAC to support the continued development and alignment of jurisdictional haemovigilance reporting systems with the recommended national haemovigilance dataset. The Australian National Haemovigilance Data Dictionary (ANHDD) was developed by the HAC to standardise the data for the national haemovigilance dataset. The ANHDD is in its third iteration and is under continuous review.
Figure 1 shows a representation of the jurisdictions contributing haemovigilance data to the current report. Validated jurisdictional‑level data was submitted by NSW, VIC, QLD, SA, TAS, the ACT and the NT. QLD contributed 2011–12 data only. WA is the only jurisdiction which did not contribute to the national dataset for the reporting period 1 July 2011 to 30 June 2013.
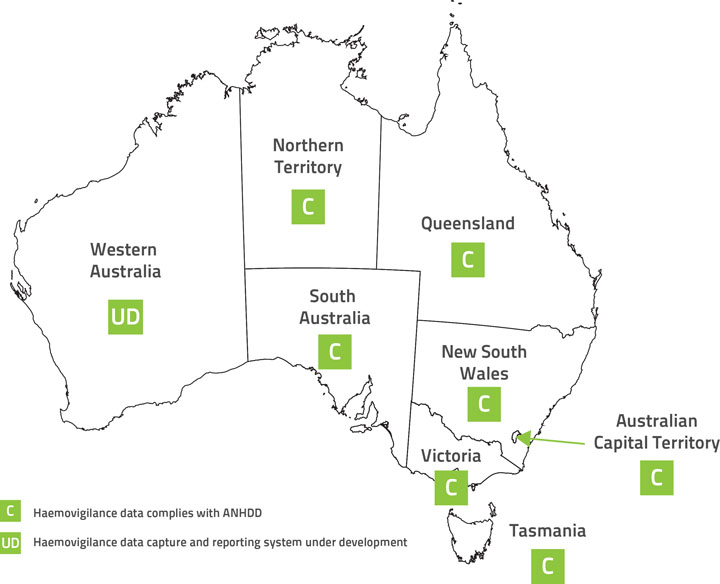
Figure 1: Jurisdictions contributing haemovigilance data to this report
Note: QLD contributed 2011–12 data only.
Victoria, Tasmania, Australian Capital Territory and Northern Territory
- VIC and TAS have supplied validated state level haemovigilance data to the National Haemovigilance Program since 2008–09 and the ACT and NT have contributed since 2009–10. The data provided by these states and territories is fully compliant with the data elements specified in the ANHDD.
South Australia
- The SA BloodSafe program has supplied validated state level haemovigilance data to the National Haemovigilance Program since 2008–09. SA recently made the ANHDD data elements such as age, sex and date of birth mandatory in the Datix Safety Learning System (SLS) to improve the completeness of data for national haemovigilance reporting.
Queensland
- The QLD Blood Management Program (QBMP) supplied validated jurisdictional‑level haemovigilance data (QiiT) from 2008–09 to 2011–12; however there were a number of definitional and conceptual differences in the data. There was a discrepancy between the age categories used for QiiT and the national dataset. Table 1 shows the transformation used to map the QiiT age categories to those of the ANHDD. The decision was taken to align the ranges with a bias towards increasing the age category. For example, the 20–29 years QiiT category has been coded as 25–34 years in the national haemovigilance dataset. This allowed re‑coding of the 28 day–1 year QiiT category and aligned with the concept that transfusion is more likely associated with increased age. De‑identification of patient data at the QiiT level made it impractical to recode every incident from the original patient records according to national haemovigilance dataset standards.
| QiiT patient age | Re-coded to the national haemovigilance dataset patient age |
|---|---|
| 28 days–1 year | 0–4 years |
| 1–4 years | 0–4 years |
| 5–9 years | 5–14 years |
| 10–19 years | 15–24 years |
| 20–29 years | 25–34 years |
| 30–39 years | 35–44 years |
| 40–49 years | 45–54 years |
| 50–59 years | 55–64 years |
| 60–69 years | 65–74 years |
| 70–79 years | 75 years or older |
| > 80 years | 75 years or older |
- The ongoing supply of QLD data to the National Haemovigilance Program has now become a major issue due to the cessation of the centralised haemovigilance system (QiiT). As a result, QLD 2012–13 data was not available for this report. The NBA has provided assistance to QLD Health to develop the Haemovigilance Data Collection Tool. QLD Heath has used the tool to improve reporting capacity for future reports.
New South Wales
- NSW has contributed to the National Haemovigilance Program by performing a targeted analysis of transfusion-related adverse events as reported in IIMS since 2008–09. However, the IIMS is not a specific haemovigilance reporting system and many important data fields required by the national haemovigilance dataset are lacking for national level reporting. As a result, the NSW data provided for the previous reports was not comparable with the data provided by other states and territories. NSW has improved the haemovigilance reporting capacity since the last report and provided detailed and validated state level data (such as imputability and outcome severity data) for this report.
Western Australia
- Adverse event data in WA is collected and analysed on an individual hospital or health service basis and was not contributed to this or previous reports. WA is developing a reporting tool and process for the collection of haemovigilance data aligned with the ANHDD. Implementation is intended to facilitate the generation of state-level haemovigilance reports and provision of WA data for national reporting.
Data quality
States and territories are primarily responsible for the quality of adverse event data provided to the National Haemovigilance Program. Transfusion-related adverse events should be validated at the local level. Standards for validation are developed by local institutions in conjunction with health departments. Reports of serious adverse events may go through a secondary validation process within the state and territory haemovigilance programs and health department quality units to ensure data accuracy and completeness. State and territory haemovigilance representatives, on behalf of health departments, will aggregate and de‑identify data and send periodic reports to the NBA. The NBA checks the validity and completeness of the reported values. Potential errors are queried with states and territories. Corrections and resubmissions may be made in response to the data queries. The NBA does not adjust data to account for possible missing or incorrect values.
- There is variation between states and territories in the quality and completeness of adverse event data reported to the National Haemovigilance Program due to the voluntary nature of reporting. Data is not complete for every reported adverse event in the national dataset and even missing for some data elements:
- NSW, VIC, QLD, SA, TAS, ACT and NT supplied validated data.
- WA did not contribute data.
- QLD data is unavailable for 2012–13.
- Sex and facility location data is unavailable for NSW.
- Time of transfusion data is unavailable for NSW and SA.
- Contributory factors are not identified for most of the adverse events reported by QLD and SA.
- The adverse events definitions standardised in the ANHDD are consistent with the IHN/ISBT definitions.
- A report is included for each adverse event, not for each patient. Patients who experienced a transfusion-related adverse event more than once may be associated with more than one report.
- In line with internationally reported trends, the Australian national haemovigilance dataset suggests that some adverse events, such as TACO, TRALI, and DHTR, are under-reported.
- Near miss data is not presented in the report. However, some states and territories, such as VIC, SA, ACT, NT and NSW, have started to collect near miss events in their systems.
- With regard to denominator data, national information on the total number of fresh blood components transfused has not been collected and reported. The NBA, states and territories are addressing this through data linkage exercises external to the National Haemovigilance Program.
