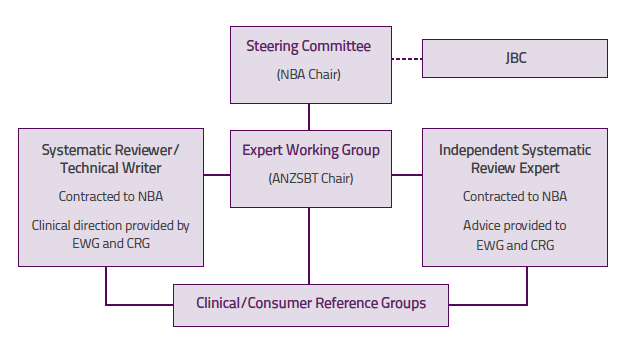
Figure A1 illustrates the management framework used to manage the development of the six modules of the guidelines.
Figure A1 Management framework for development of the guidelines
ANZSBT, Australian & New Zealand Society of Blood Transfusion; CRG, Clinical/Consumer Reference Group; EWG, Expert Working Group; GAR, Guidelines Assessment Register; JBC, Jurisdictional Blood Committee; NBA, National Blood Authority; NHMRC, National Health and Medical Research Council
A2 Terms of reference
Steering Committee
The overarching Steering Committee was established to provide coordination and direction for development
of the guidelines. It was chaired by the NBA, with representation from the ANZSBT, the NHMRC (including
a member from the National Institute of Clinical Studies), a state expert and an expert from the Australian
Government Department of Health and Ageing. The role of the Steering Committee was to:
- develop and oversee the project plan for the revision of the guidelines
- recommend the membership of the EWG to the NBA Chief Executive Officer, who will appoint the
recommended members
- endorse the scope of the project as proposed by the EWG, and the process by which it will
be undertaken
- ensure that there is effective communication and consultation with all relevant stakeholders for the
duration of the project, including the development of a communications and engagement strategy that
meets NHMRC requirements
- provide information through the NBA to the Jurisdictional Blood Committee (JBC) on the project
- review resources that are dedicated to the project, to ensure that they are sufficient for the project to
meet its deadlines
- review and approve revisions to the project plan and terms of reference
- address other matters as raised by members of the Steering Committee or EWG.
Expert Working Group
The EWG was formed to advise the Steering Committee about the scope and structure of the guidelines,
and to determine the focus of the systematic review of the evidence-based literature. The group’s terms of
reference were:
- to consider the scope of the project and proposed structure of the guidelines, as referred by the Steering
Committee and, if necessary, to present recommendations for revisions to the Steering Committee
- under the guidance of the NHMRC GAR expert, to formulate the clinical questions to be answered by the
literature review
- to provide clinical oversight for the development of the content of the guidelines, in particular,
ensuring that:
– the research undertaken is comprehensive
– the quality of the revised guidelines will meet with clinical approval
- to provide recommendations on the terms of reference for the CRGs and oversee coordination of the
activities of the CRGs
- to ensure appropriate engagement by consumers at all relevant points
- to assist in the development or review of tools and strategies to support the implementation and audit
of the guidelines and review their uptake
- to facilitate consultation and the uptake of the guidelines
- to respond to any additional requirements to ensure compliance with the NHMRC guidelines
development processes.
A2 Terms of reference
Systematic reviewers and technical writers
The NBA contracted systematic reviewers and technical writers to conduct systematic reviews of the scientific literature and provide technical writing services to produce each module and associated deliverables, including technical reports.
Clinical/Consumer Reference Groups
A CRG was formed to review the module during development and, with the assistance of technical writers, to formulate recommendations aimed at optimising patient blood management based on systematic review findings, or, in the absence of evidence, to develop practice points through a consensus-based process. The CRG also provided advice to the EWG on guideline relevance and utility for targeted service providers and recipients who will use or benefit from the guidelines. Pertinent terms of reference for guidelines development included:
- the CRGs may review and offer advice on the set of questions to be put to the systematic review
for the project
- the CRGs may review the draft guidelines and consumer materials, and offer advice on the way information is presented in terms of relevance and utility to the groups they represent
- the CRGs will not have authority or decision-making power over how that advice is used.
Guidelines Assessment Register expert
Two GAR experts were appointed by the NHMRC to provide advice and mentoring to the EWG and CRG, and to ensure that the new guidelines and the development process implemented by each reference group complied with NHMRC requirements.
Clinical/Consumer Reference Group for Critical Bleeding/Massive Transfusion
|
A/Prof Larry McNicol (Chair)
Anaesthetist
Australian & New Zealand College of Anaesthetists
|
|
Prof Zsolt Balogh
Trauma surgeon
Royal Australasian College of Surgeons
|
|
Mr Shannon Farmer
Consumer
Independent consumer advocate
|
|
Dr Craig French
Intensive care physician
College of Intensive Care Medicine of Australia and New Zealand and Australian & New Zealand Intensive Care Society
|
|
Prof Russell Gruen
Trauma Surgeon
Royal Australasian College of Surgeons
|
|
Dr Chris Hogan
Haematologist
National Blood Authority
|
|
Dr Richard Seigne
Anaesthetist
Australian & New Zealand Society of
Blood Transfusion
|
|
Mr Daryl Teague
Orthopaedic surgeon
Australian Orthopaedic Association
|
|
Dr Amanda Thomson
Haematologist
Australian & New Zealand Society of
Blood Transfusion
|
|
Dr Philip Truskett
Surgeon
Royal Australasian College of Surgeons
|
|
Dr John Vinen
Emergency care physician
Australasian College for Emergency Medicine
|
National Health and Medical Research Council appointed Guidelines
Assessment Register consultants
| Ms Tracy MerlinAdelaide Health Technology Assessment (AHTA), University of Adelaide |
| Ms Skye Newton Adelaide Health Technology Assessment (AHTA), University of Adelaide |
Project Management and Committee Secretariat – provided by the NBA
| Ms Leia EarnshawProject Officer, Blood Sector Clinical Development |
| Dr Paul HylandAssistant Director, Blood Sector Clinical Development |
| Dr Dejan KrstikAssistant Director, Blood Sector Clinical Development |
| Ms Jennifer RobertsDirector, Blood Sector Clinical Development |
Systematic review team for critical bleeding/massive transfusion
| Dr Jane AdamsIMS Health Australia (Engagement Manager, Health Outcomes) |
| Ms Miranda BaileyIMS Health Australia (Senior Consultant, Health Outcomes) |
| Mr Laurence FongIMS Health Australia (Principal, Pricing and Market Access) |
| Dr John GillespieIMS Health Australia (Engagement Manager, Health Outcomes) |
| Mr Alasdair GodfreyIMS Health Australia (Engagement Manager,, Health Outcomes) |
| Ms Ann JonesIMS Health Australia (Senior Medical Editor, Health Outcomes) |
| Dr Jodie WilsonIndependent contractor to IMS Health Australia |
| Ms Teresa WozniakIMS Health Australia (Consultant, Health Outcomes) |
Medical writing (Guideline only) and technical editing – Health Technology Analysts
| Dr Suzanne CampbellHealth Technology Analysts (Health Outcomes Manager) |
| Dr Adele WestonHealth Technology Analysts (Director) |
| Dr Hilary CadmanCadman Editing Services (independent contractor to Health Technology Analysts) |
A4 Conflict of interest
All members of the Steering Committee, CRG and EWG declared any conflicts of interest before starting work on the guidelines. Conflicts of interest were reviewed at intervals during the development of the guidelines and required to be declared at the commencement of each meeting.
A5 Acknowledgements
The CRG thanks the following facilities, whose MTPs were considered in developing the template MTP:
- Australia
– Northern Sydney Central Coast Area Health Service
– Queensland Blood Products Advisory Committee
– Royal Perth Hospital, Western Australia
– Royal Adelaide Hospital, South Australia
– Sydney South West Area Health Service
– The Alfred, Victoria
- New Zealand
– Auckland District Health Board
– Canterbury District Health Board
The CRG thanks the Haemostasis Registry, Department of Epidemiology and Preventive Medicine, Monash University for providing access to their data on rFVIIa.