Appendix A Governance
A1 Management framework for guideline development
Figure A1 illustrates the framework used to manage the development of the six modules of the guidelines, described in Section 1.2 of Chapter 1. Their roles and responsibilities are outlined in A2 Terms of Reference.
Figure A1 Management framework for development of the guidelines
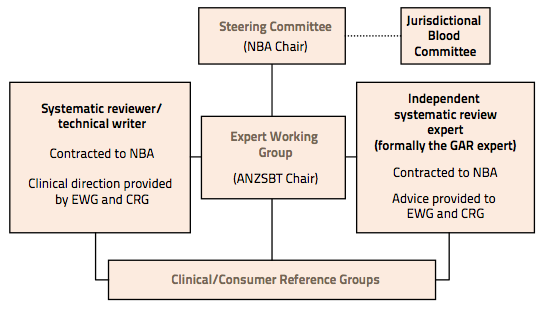
ANZSBT, Australian & New Zealand Society of Blood Transfusion; CRG, Clinical/Consumer Reference Group; EWG, Expert Working Group; GAR, NHMRC Guidelines Assessment Register; NBA, National Blood Authority
A2 Terms of reference
Steering Committee
The overarching Steering Committee was established to provide coordination and direction for development of the guidelines. It was chaired by the NBA, with representation from the ANZSBT the Jurisdictional Blood Committee and a clinical representative from the Australian Government Department of Health and Ageing. The role of the Steering Committee was to:
- develop and oversee the project plan for the revision of the guidelines
- recommend the membership of the EWG to the NBA Chief Executive Officer, who will appoint the recommended members
- endorse the scope of the project as proposed by the EWG, and the process by which it will be undertaken
- ensure that there is effective communication and consultation with all relevant stakeholders for the duration of the project, including the development of a communications and engagement strategy that meets NHMRC requirements
- provide information on the project through the NBA to the JBC
- review resources that are dedicated to the project, to ensure that they are sufficient for the project to meet its deadlines
- review and approve revisions to the project plan and terms of reference
- address other matters as raised by members of the Steering Committee or EWG.
Expert Working Group
The EWG was formed to advise the Steering Committee about the scope and structure of the guidelines, and to determine the focus of the systematic review of the evidence-based literature. The group’s terms of reference were to:
- consider the scope of the project and proposed structure of the guidelines as referred by the Steering Committee, and, if necessary, to present recommendations for revisions to the Steering Committee
- under the guidance of the NHMRC independent systematic review expert, formulate the clinical questions to be answered by the literature review
- provide clinical oversight for the development of the content of the guidelines, in particular, ensuring that:
- the research undertaken is comprehensive
- the quality of the revised guidelines will meet with clinical approval
- provide recommendations on the terms of reference for the CRGs and oversee coordination of the activities of the CRGs
- ensure appropriate engagement by consumers at all relevant points
- assist in the development or review of tools and strategies to support the implementation and audit of the guidelines and review their uptake
- facilitate consultation and uptake of the guidelines
- respond to any additional requirements to ensure compliance with the NHMRC guidelines development processes.
Systematic reviewers and technical writers
The NBA contracted systematic reviewers and technical writers to conduct systematic reviews of the scientific literature and provide technical writing services to produce each module and technical reports.
Clinical/Consumer Reference Groups
A CRG was formed to review each phase of the guidelines during development. With the assistance of technical writers, the CRGs formulated recommendations aimed at optimising patient blood management based on systematic review findings, or, in the absence of evidence, developed practice points through a consensus-based process. The CRGs also provided advice to the EWG on guideline relevance and utility for targeted service providers and recipients who will use or benefit from the guidelines. Pertinent terms of reference for guidelines development included the following:
- the CRGs may review and offer advice on the set of questions to be put to the systematic review for the project
- the CRGs may review the draft guidelines and consumer materials, and offer advice on the way information is presented in terms of relevance and utility to the groups they represent
- the CRGs will not have authority or decision-making power over how that advice is used.
A3 Membership of bodies involved in governance of the guidelines
Steering Committee
| Name | Organisation |
|---|---|
| Ms Stephanie Gunn (Chair) | National Blood Authority |
| Mr Ken Davis | Australian & New Zealand Society of Blood Transfusion |
| Prof Henry Ekert | Australian Government Department of Health and Ageing |
| Ms Sue Ireland | Jurisdictional Blood Committee |
| Dr Amanda Thomson | Australian & New Zealand Society of Blood Transfusion |
Expert Working Group
| Name | Organisation |
|---|---|
| Dr Craig French (Co-chair) | College of Intensive Care Medicine of Australia and New Zealand, and Australian & New Zealand Intensive Care Society |
| Dr Amanda Thomson (Co-chair) | Australian & New Zealand Society of Blood Transfusion |
| A/Prof Donald Bowden | Thalassaemia Australia |
| A/Prof Mark Dean | Haematology Society of Australia and New Zealand & Royal Australasian College of Physicians |
| Mr Shannon Farmer | Patient Blood Management Advocate |
| Dr Chris Hogan | National Blood Authority |
| Ms Janine Learmont | Royal College of Nursing, Australia |
| Dr Helen Liley | Royal Australasian College of Physicians, Paediatric & Child Health Division |
| Dr Robert Lindeman | Royal College of Pathologists of Australasia |
| A/Prof Larry McNicol | Australian & New Zealand College of Anaesthetists |
| Prof Michael Permezel | Royal Australian & New Zealand College of Obstetricians and Gynaecologists |
| Dr Kathryn Robinson | Australian Red Cross Blood Service |
| Dr Richard Seigne | Australian & New Zealand Society of Blood Transfusion |
| Dr Philip Truskett | Royal Australasian College of Surgeons |
| Dr John Vinen | Australasian College for Emergency Medicine |
Clinical/Consumer Reference Group – Medical Module
| Name | Specialty | Organisation |
|---|---|---|
| A/Prof Mark Dean (Chair) | Haematologist | Royal College of Physicians & Haematology Society of Australia & New Zealand |
| Dr Lilon Bandler | General practitioner and Indigenous health representative | Royal Australian College of General Practitioners |
| A/Prof Donald Bowden | Haematologist | Thalassemia Australia |
| Prof John Duggan* | Gastroenterologist | Independent expert – gastroenterology |
| Mr Shannon Farmer | Researcher | Patient Blood Management Advocate |
| Dr Craig French | Intensive care physician | College of Intensive Care Medicine of Australia and New Zealand, and Australian & New Zealand Intensive Care Society |
| Dr Chris Hogan | Haematologist | National Blood Authority |
| Dr Robert Lindeman | Haematologist | Royal College of Pathologists Australia |
| Prof Lawrence McMahon* | Nephrologist | Independent expert – renal medicine |
| Ms Penny O’Beid | Clinical Nurse Consultant, Transfusion Medicine | Royal College of Nursing Australia |
| Dr Kathryn Robinson | Haematologist | Australian Red Cross Blood Service |
| Dr Amanda Thomson | Haematologist | Australian & New Zealand Society of Blood Transfusion |
* Two members joined the CRG for the final four of 12 meetings after the review of the evidence and formulation of recommendations. This additional membership was sought to provide specialist input for specific populations (i.e. renal medicine and gastroenterology) and to ensure that the guidance developed by the CRG accorded, in so far as the evidence allowed, with other guidelines for these specific populations
Background research
| Name | Specialty | Organisation |
|---|---|---|
| Dr Nina Dhondy | Haematology Registrar | Royal North Shore Hospital, Sydney |
| Dr Chris Hogan | Haematologist | National Blood Authority |
| Dr Robert Lindeman | Haematologist | Royal College of Pathologists Australia |
| Dr Amanda Thomson | Haematologist | Australian & New Zealand Society of Blood Transfusion |
Acknowledgements – additional clinical input
| Name | Specialty | Organisation |
|---|---|---|
| A/Prof Jane Andrews | Gastroenterologist | Royal Adelaide Hospital |
| Dr Jeffrey Roland | Geriatrician | The Prince Charles Hospital |
| Dr Jenny Shannon | Oncologist | Nepean Cancer Care Centre |
Independent systematic review expert
| Name | Organisation |
|---|---|
| Ms Tracy Merlin | Adelaide Health Technology Assessment (AHTA), University of Adelaide |
Project Management and Committee Secretariat – provided by the NBA
| Name | Organisation |
|---|---|
| Ms Leia Earnshaw | A/g Assistant Director, Blood Sector Clinical Development |
| Dr Paul Hyland | Assistant Director, Blood Sector Clinical Development |
| Ms Jennifer Roberts | Director, Blood Sector Clinical Development |
Systematic review team
| Name | Organisation |
|---|---|
| Ms Nimita Arora | OptumInsight (Senior Project Leader) |
| Dr Kristina Coleman | OptumInsight (Principal Analyst) |
| Dr Briony Jack | OptumInsight (Research Analyst) |
| Mr Gregory Merlo | OptumInsight (Senior Analyst) |
Medical writing and technical editing – OptumInsight
| Name | Organisation |
|---|---|
| Dr Hilary Cadman | Cadman Editing Services (independent contractor to OptumInsight) |
A4 Conflict of interest
All members of the Steering Committee, CRG, EWG and systematic review team declared any interests before starting work on the guidelines. Interests were also reviewed at intervals, and were required to be declared at the start of each meeting. The NBA keeps a register of all declared interests. If an interest is declared, the CRG decide by consensus if it affects the proceedings. If the interest is considered to be competing or in conflict, the Chair can prevent the member from participating in discussions and decisions pertaining to the declared interest. Three members declared interests during the guideline development process. Mr Shannon Farmer declared the following patient advocacy roles: the Society for the Advancement of Blood Management, the Medical Society for Blood Management and the Network for Advancement of Transfusion Alternatives. Professor Lawrence McMahon declared that he was a prescriber of erythropoiesis stimulating agents. He declared travel grants to attend the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) Annual Scientific Meeting in 2010 from Roche and in 2012 from Amgen. He received a research grant from Amgen in 2009 and an unrestricted educational grant for research from Roche in 2011. He was on the Roche Advisory Board for Mircera (continuous erythropoietin receptor activator) in 2008. Dr Kathryn Robinson declared an interstate airfare and accommodation for one night paid directly by Aspen Pharmacare for presenting at an educational iron forum organised by Aspen in February 2008; information from her presentation was used for an Aspen educational newsletter but no payment was received.
The chair considered these declarations and determined that they did not constitute a sufficient conflict to require members to leave the room or excuse themselves from discussion at any time during the guideline development process. No other members declared any interests.