Summary of recommendations and practice points
The CRG developed recommendations where sufficient evidence was available from the systematic review of the literature. The recommendations have been carefully worded to reflect the strength of the body of evidence. Each recommendation has been given a grade, using the following definitions, set by the NHMRC:
GRADE A |
Body of evidence can be trusted to guide practice |
|---|---|
GRADE B |
Body of evidence can be trusted to guide practice in most situations |
GRADE C |
Body of evidence provides some support for recommendation(s), but care should be taken in its application |
GRADE D |
Body of evidence is weak and recommendations must be applied with caution. |
The CRG developed practice points where the systematic review found insufficient high-quality data to produce evidence-based recommendations, but the CRG felt that clinicians require guidance to ensure good clinical practice. These points are based on consensus among the members of the committee.
Appendix F gives the recommendations and practice points by clinical condition.
Recommendations
| Conditions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifier and Grade |
Guidance |  |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
| R1 Grade C |
In ACS patients with a Hb concentration >100 g/L, RBC transfusion is not advisable because of an association with increased mortality. | 3.2.2 | ||||||||||
| R2 Grade A |
In cancer patients with anaemia, the routine use of ESAs is not recommended because of the increased risks of mortality and thromboembolic events. | 3.3.1 | ||||||||||
| R3 Grade B |
In patients with CHF, identification and treatment of iron deficiency (absolute and functional) is recommended to improve functional or performance status. This is consistent with the 2011 update to the Guidelines for the Prevention, Detection and Management of Chronic Heart Failure in Australia, 2006.2 Note: The studies reviewed only included patients treated with IV iron, and of NYHA functional classes II or III. |
3.3.2 | ||||||||||
| R4 Grade B |
In anaemic patients with CKD, ESA therapy to a low to intermediate Hb target may be used to avoid RBC transfusion, after consideration of risks and benefits for the individual patient (Grade B). Note: The CARI guidelines recommend a Hb target between 100-115 g/L5 |
3.3.3 | ||||||||||
| R5 Grade C |
In anaemic patients with CKD, ESA therapy to a low to intermediate Hb target may be used to relieve fatigue, after consideration of risks and benefits for the individual patient (Grade C). Note: The CARI guidelines recommend a Hb target between 100-115 g/L5 |
3.3.3 | ||||||||||
| R6 Grade B |
In anaemic patients with CKD, ESA therapy to a Hb target of over 130 g/L is not recommended because of increased morbidity. | 3.3.3 | ||||||||||
| R7 Grade B |
In anaemic patients with non dialysis-dependent CKD, type 2 diabetes and a history of malignancy, the routine use of ESAs is not recommended because of the increased risk of cancer- related mortality. | 3.3.3 | ||||||||||
| R8 Grade B |
In patients undergoing chemotherapy and haematopoietic stem cell transplantation, the recommended strategy for prophylactic use of platelets is transfusion at a platelet count of <10 × 109/L in the absence of risk factors, and at <20 × 109/L in the presence of risk factors (e.g. fever, minor bleeding). |
3.5.3 | ||||||||||
ACS, acute coronary syndrome; CHF, chronic heart failure; CKD, chronic kidney disease; ESA, erythropoiesis-stimulating agent; Hb, haemoglobin; IV, intravenous; NYHA, New York Heart Association; R, recommendation; RBC, red blood cell; TTP, thrombotic thrombocytopenic purpura
Practice points
| Conditions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifier | Guidance |  |
 |
 |
 |
 |
 |
 |
 |
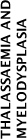 |
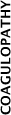 |
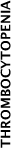 |
| PP1 | RBC transfusion should not be dictated by a Hb concentration alone, but should also be based on assessment of the patient’s clinical status. | 3.2.1 | ||||||||||
| PP2 | Where indicated, transfusion of a single unit of RBC, followed by clinical reassessment to determine the need for further transfusion, is appropriate. This reassessment will also guide the decision on whether to retest the Hb level. | 3.2.1 | ||||||||||
| PP3 |
Direct evidence is not available in general medical patients.a Evidence from other patient groups and CRG consensus suggests that, with a:
a Recommendations and practice points for medical patients in a critical care setting will be found in the Patient Blood Management Guidelines: Module 4 – Critical Care.3 Recommendations and practice points for specific medical subgroups (ACS, CHF, cancer, acute upper gastrointestinal bleeding and chronically transfused) appear elsewhere in this module. |
3.2.1 | ||||||||||
| PP4 | In patients with iron deficiency anaemia, iron therapy is required to replenish iron stores regardless of whether a transfusion is indicated. | 3.2.1 | ||||||||||
| PP5 | In patients with ACS and a Hb concentration <80 g/L, RBC transfusion may be associated with reduced mortality and is likely to be appropriate. (See PP1 and PP2). | 3.2.2 | ||||||||||
| PP6 | In patients with ACS and a Hb concentration of 80 – 100 g/L, the effect of RBC transfusion on mortality is uncertain and may be associated with an increased risk of recurrence of MI. Any decision to transfuse should be made with caution and based on careful consideration of the risks and benefits. (See PP1 and PP2). | 3.2.2 | ||||||||||
| PP7 | In all patients with heart failure, there is an increased risk of transfusion-associated circulatory overload. This needs to be considered in all transfusion decisions. Where indicated, transfusion should be of a single unit of RBC followed by reassessment of clinical efficacy and fluid status. For further guidance on how to manage patients with heart failure, refer to general medical or ACS sections, as appropriate (R1, R3, PP3–PP6). | 3.2.3 | ||||||||||
| PP8 | In patients with cancer, the aetiology of anaemia is often multifactorial; where appropriate, reversible causes should be identified and treated. | 3.2.4 3.3.1 |
||||||||||
| PP9 | There is a lack of specific evidence relating to the effects of RBC transfusion in patients with cancer. Any decision to transfuse should be based on the need to relieve clinical signs and symptoms of anaemia. When treating patients with cancer, refer also to the general medical population PP1–PP4. | 3.2.4 | ||||||||||
| PP10 | In well-compensated patients with acute upper gastrointestinal blood loss that is non-critical, there is no evidence to favour a liberal transfusion policy. Therefore, a more restrictive approach may be appropriate. There are no data to support a specific Hb treatment target in these patients. | 3.2.5 | ||||||||||
| PP11 | For critically bleeding patients, refer to Patient Blood Management Guidelines: Module 1 – Critical Bleeding/Massive Transfusion (2011).4 | 3.2.5 | ||||||||||
| PP12 | In anaemic patients with cancer receiving ESAs, evaluate iron status to guide adjuvant iron therapy. | 3.3.1 | ||||||||||
| PP13 | ESA use is less effective in patients with chronic renal failure who have absolute or functional iron deficiency. | 3.3.3 | ||||||||||
| PP14 | For comprehensive information about ESA and iron therapy in patients with CKD, refer to CARI iron guidelines.5 | 3.3.3 | ||||||||||
| PP15 | In patients with IBD, determine the cause of anaemia and treat reversible causes. IV iron may be required in patients who are intolerant of oral iron, or to avoid aggravation of intestinal inflammation. | 3.3.5 | ||||||||||
| PP16 |
The routine use of FFP in medical patients with coagulopathy (including those with liver impairment) is not supported. Tests for coagulation correlate poorly with bleeding risk in liver impairment. The underlying causes of coagulopathy should be assessed. Where FFP transfusion is considered necessary, the risks and benefits should be considered for each patient, and expert guidance sought. |
3.4.1 | ||||||||||
| PP17 |
For guidance on the use of FFP in specific patient groups, refer to:
|
3.4.1 | ||||||||||
| PP18 | The routine use of cryoprecipitate or fibrinogen concentrate in medical patients with coagulopathy is not advised. The underlying causes of coagulopathy should be identified; where transfusion is considered necessary, the risks and benefits should be considered for each patient. Specialist opinion is advised for the management of DIC. | 3.4.2 | ||||||||||
| PP19 |
For guidance on the use of cryoprecipitate or fibrinogen concentrate in specific patient groups, refer to:
|
3.4.2 | ||||||||||
| PP20 | Platelet transfusion may be indicated for the prevention and treatment of haemorrhage in patients with thrombocytopenia or platelet function defects. Platelet transfusions are not indicated in all causes of thrombocytopenia, and may be contraindicated in certain conditions (e.g. TTP and HIT). Thus, the cause of the thrombocytopenia should be established and expert opinion sought. | 3.4.3 | ||||||||||
| PP21 |
In patients with chronic failure of platelet production (e.g. myelodysplasia or aplastic anaemia), a specific threshold for transfusion may not be appropriate. These patients are best managed on an individual basis, in consultation with a relevant expert.9 Long-term prophylactic platelet transfusions may be best avoided because of the risk of complications (e.g. alloimmunisation and platelet refractoriness). Therapeutic platelet transfusions could be considered for treatment of bleeding. |
3.4.3 | ||||||||||
| PP22 |
In patients undergoing chemotherapy and haematopoietic stem cell transplantation, there is no evidence to support:
Further research to determine the safety and efficacy of a lower platelet transfusion trigger is underway. |
3.5.3 | ||||||||||
| PP23 | In patients with thalassaemia, the evidence does not support any change to the current practice of maintaining a pretransfusion Hb concentration of 90 – 100 g/L, with transfusions at about monthly intervals. | 3.6.1 | ||||||||||
| PP24 | In patients with myelodysplasia who are regularly and chronically transfused, there is no evidence to guide particular Hb thresholds. Decisions around appropriate triggers and frequency of transfusion need to be individualised, taking into account anaemia-related symptoms, functional or performance status, and the patient’s response to previous transfusions. | 3.6.2 | ||||||||||
ACS, acute coronary syndrome; AHCDO, Australian Haemophilia Centre Directors’ Organisation; CARI, Caring for Australasians with Renal Impairment; CHF, chronic heart failure; CKD, chronic kidney disease; CRG, Clinical/Consumer Reference Group; DIC, disseminated intravascular coagulation; ESA, erythropoiesis-stimulating agent; FFP, fresh frozen plasma; Hb, haemoglobin; HIT, heparin-induced thrombocytopaenia; IBD, inflammatory bowel disease; IV, intravenous; MI, myocardial infarction; PP, practice point; R, recommendation; RBC, red blood cell; TTP, thrombotic thrombocytopenic purpura