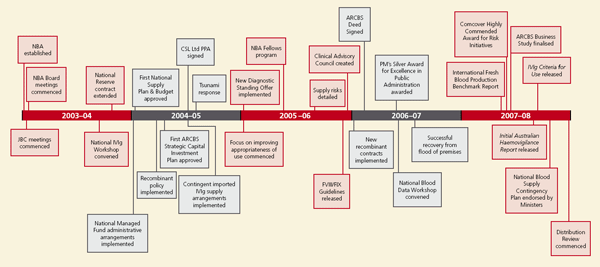
1.4 The first five years
Five years after the establishment of the NBA, meaningful improvements in the Australian blood sector are now in place.
The challenges in the first year were considerable — not the least being the task of creating an organisation from scratch and tackling the heady expectations arising from the National Blood Agreement. Other challenges included:
- developing and managing new relationships at a national level
- building confidence in the clinical and scientific communities about the roles and responsibilities of the new authority
- demonstrating value for governments early in our existence.
Today, the NBA negotiates and manages contracts covering all the blood and blood-related products used in Australia. We also have a broad international network; coordinate a national approach to blood supply, usage and conservation; manage a diversity of stakeholder relationships; and have recently started work to drive improvements in transfusion appropriateness and blood sector performance analysis.
In the five years of operation to date, the NBA has:
- improved contract management, delivering significant cost savings to all Australian governments
- implemented a diverse and innovative range of supply contingency provisions that have prevented significant stock shortages
- established clear governance decision-making frameworks that integrate blood into the overall decision-making framework for health sector risk planning
- built highly effective relationships with key stakeholders
- delivered a range of safety and quality initiatives such as the recent Initial Australian Haemovigilance Report (2008) and the Criteria for the Clinical Use of Intravenous Immunoglobulin (IVIg) in Australia (2008)
- began data collections on product issues and usage to assist in developing policies relating to appropriate use of products, and a rigorous analysis of future supply and demand
- implemented a range of strategies to reduce inappropriate use of blood products.
Several key policy challenges lie ahead for the NBA in the next several years, namely:
- the successful management of the Review of Distribution Arrangements for Blood Products
- new contracts with CSL Ltd and ARCBS
- finalising new guidelines on appropriate use of fresh blood products
- collection and management of data to understand how we use products
- future directions for patient blood management to reduce patient exposure to donor blood and ultimately deliver better outcomes for patients.

Dr Alison Turner, Ms Louise Morauta, then Chair JBC and Professor Dick Smallwood, then Chair NBA Board at the opening of the NBA premises in July 2003.
The achievements shown in Table 1.1 have been possible because of a commitment to six principles, namely:
- detailed analysis of costs, options, international comparators and stakeholder concerns
- meticulous planning of resources and activities against clear goals
- seeking creative and innovative ways to achieve better outcomes
- a high-level of stakeholder involvement and communication, both in the planning and designing phases, as well as implementation
- careful management of cautious implementation
- regular monitoring and review for all the projects undertaken.
The success that this approach produced is now allowing us to embark on a slightly more adventurous approach to our responsibilities and five years out we are looking forward to delivering on actions that will further demonstrate our leadership in the blood sector. We accept that this will be dependent on the quality of our engagement with our stakeholders, and clear, consistent and regular communication of government priorities and objectives with these stakeholders.
We look forward to these challenges and the next five years.
Table 1.1 Five years of achievements, 2003–08
| National Blood Authority Act supply obligations | Deliverable | Outcome |
To provide assistance:
|
Provided secretariat services to 53 JBC meetings. JBC focus evolved from strong operational concerns to strategic developmental focus. Board directly engaged in informing strategic advice to the General Manager. Clinical Advisory Council established to provide detailed clinical advice and was instrumental in the development of the blood counts program. |
Effective governance framework that supports consolidated policy decision-making and implementation across all jurisdictions. |
| To carry out national blood arrangements relating to annual plans and budgets for the production and supply of blood products and services. | National supply plan process created and implemented on an annual basis. Presentation of plans delivered progressively earlier to Ministers each year. Suppliers advised of indicative volumes more than six months before the commencement of plan. Mid-year reviews designed and implemented on a regular basis from 2004. Verification procedures for ordering and receipting, negotiated and implemented. |
Suppliers provided with improved certainty and clarity of government supply requirements. An improved level of accountability in blood expenditure. |
| To enter and manage contracts and arrangements for the collection, production and distribution of the blood products and services necessary to ensure a sufficient supply of blood products and services in all the states and covered territories. | New Deed with ARCBS negotiated and signed. New Deed with CSL Ltd negotiated< and signed. New contractual arrangements for diagnostics implemented. New Standing Order arrangements for the importation and implementation of IVIg. New Deeds for imported plasma and recombinant products negotiated and signed. |
No stock-out of any product in five years. Substantial savings achieved. Improved shelf life of some products. Improved product access for patients. Improved products and product range. |
| To carry out national blood arrangements to ensure that there is a sufficient supply of blood products and services in all the states and covered territories. | Effective management of the development of:
|
High-quality, evidence-based guidelines for high cost products developed in consultation with the clinical community and published. |
| To carry out national blood arrangements relating to. (a) Safety and quality measures. |
ARCBS requests to governments for funding for increased levels of leucodepletion, bacterial contamination and sample archiving assessed. Rolled out recombinant fVIII for all patients seeking this product. Obtained funding for ARCBS to implement TGA requirement for changes to haemoglobin tests. Blood standard requirements integrated into hospital accreditation reform. |
Product recipients have access to a selection of some of the world’s best products. Government approved 100% leucodepletion and 100% bacterial contamination testing of platelets. |
| (b) Contingency measures and risk mitigation measures for the supply of blood products and services. | Agreed National Blood Supply Contingency Plan. Risk mitigation strategies incorporated into plasma and recombinant products contracts. New management arrangements for National Managed Fund instigated. In-country reserve requirements. |
Effective coordination of national management of blood product shortages. Blood sector integrated into health contingency planning. Hierarchy of risk response options. |
| To liaise with and gather information from governments, suppliers and others about matters relating to blood products and services. | Ongoing horizon scanning project implemented. Fresh Blood Products: Production Benchmarking and Demand Drivers released. Strategic workshops initiated for JBC. Created Blood Suppliers Forum, Professional and Community Forum and the CAC. Engaged stakeholders in specific projects, eg ABDR redevelopment, NIMS and product tender processes. Established haemovigilance project working group. Established an EWG to manage the review of guidelines for use of fresh blood components. |
All contracts and major projects informed by international research, government, clinical and community needs. |
| To provide information and advice to the Minister and the Ministerial Council about matters relating to blood products and services. | Approximately 20 papers have been submitted to AHMC for ministerial approval. Recommended position on barcoding policy for blood products. |
Ministers provided with consolidated strategic national policy and funding advice. Ministers provided with accurate and timely advice. |
| To carry out national blood arrangements relating to the funding of the supply of blood products and services, and the NBA’s operations. | Established the NBA with independent infrastructure and services, including IT systems; outsourcing of noncore business systems; appropriate and best practice governance arrangements; and key policies and processes to support the operation of the NBA. Maintained an active recruitment program to address new functions. Implemented an NBA Fellows program to ensure the NBA has access to high-quality advice. Implemented CAC to ensure the NBA has access to high level clinical advice to guide policy and program development. Recognition as high performing agency through:
|
NBA recognised for public service excellence. NBA recognised for high quality risk management procedures. |
| National Blood Authority Act — Safety & Quality obligations |
Deliverable | Outcome |
| Ensure that funding and supply contracts for the national blood supply include appropriate obligations on suppliers to meet safety and quality standards and to enforce those obligations. | All contracts comply with this requirement.
Requirement for additional pathogen inactivation steps in some CSL Ltd products in new PPA. Co-ordinated supply response for implementation of vCJD mitigation steps. |
Australian supply contracts provide some of the world’s best products. |
| Maintain a systematic approach to identify new developments and to provide a clearinghouse and coordination function for information in relation to new developments. | Framework for assessment of new technologies established.
Supply contracts to provide information on new and emerging issues. Blood Sector Data Strategy |
Framework to ensure that the sector is informed and well poised for timely responses to new and emerging pressures.
Standardised approach to information management and data systems development in the blood sector to be implemented. |
| Facilitate coordination, integration, cooperation and information exchange between the NBA and other bodies with a safety and quality role in the Australian blood sector, and between those other bodies. | Coordinated blood sector input to National Safety and Quality in Healthcare Commission accreditation reforms.
Provided input to the Information Strategy for the National Safety and Quality in Healthcare Commission. Designed ABDR with input from NEHTA. Red Cell Utilisation Workshop sharing data between states. Co-sponsored support for market research on red cell prescribing behaviours of doctors (with NSW Government). |
Blood issues integrated into health S&Q reform agenda. |
| Provide information and advice to the JBC and to the Ministerial Council (through the JBC). | Provided information on new and emerging products, technologies and procedures to JBC on a regular basis.
Established strategic workshops for JBC. Prepared more than 500 papers to JBC. |
Sound coverage and management of operational issues.
Policy directions to be informed by careful consideration of emerging information. |
| Act on behalf of the JBC to purchase and organise activities under Clauses 34 (a), (b) and (c). | Started distribution review.
Established a better practice fund to develop local ideas to national application. |
Four projects commenced under this fund. |
| Facilitate the development of national information systems for S&Q issues in relation to the Australian blood sector. | Haemovigilance reporting design requirements.
Australia’s first haemovigilance report to guide sector reform. ABDR redevelopment to provide improved demand data. |
Awareness of transfusion-related reactions increased.
Australia now meets best international practice in haemovigilance. High-quality, evidence- based guidelines for high cost products developed in consultation with the clinical community and published. |
ABDR = Australian Bleeding Disorders Registry; AHMC = Australian Health Ministers’ Conference; ARCBS = Australian Red Cross Blood Service; CAC = Clinical Advisory Council; EWG = expert working group; GM = general manager; JBC = Jurisdictional Blood Committee; NBA = National Blood Authority; NEHTA = National E-Health Transition Authority; NMF = National Managed Fund; PPA = Plasma Products Agreement; S&Q = safety and quality; TGA = Therapeutic Goods Administration; vCJD = variant Creutzfeldt-Jakob disease.
National Blood Authority – First Five Years Highlights
[Click image above for larger version]
< Previous | Contents | Next >