Part Five: Future trends in blood management and horizon scanning
Download Part 5: Future trends in blood management and horizon scanning as a PDF (150kb)
Part Five explores current and future developments both nationally and internationally that may in future influence the management of blood and blood-related products in Australia.
The National Blood Authority (NBA) has a function under the National Blood Authority Act 2003 to liaise with, and gather information from, governments, suppliers and others about matters relating to blood products and services. This function supports two of the governments’ secondary objectives under Clause 2 of the National Blood Agreement, which are to:
- monitor the national and international environment in which the Australian blood sector operates for new technological, clinical, risk or other developments that may impact on the national blood supply
- undertake national information gathering, monitoring of new developments, reporting and research in relation to the Australian blood sector.
To monitor international developments that may influence the management of blood and blood-related products in Australia, our focus is on:
- information that may have an impact on global supply, demand and pricing such as changes in company structure, capacity, organisation and ownership
- potential new product developments and applications
- global regulatory and blood practice trends
- other emerging risks that could potentially put financial or other pressures on the Australian sector.
As part of this work, the NBA started a Review of New Technologies, New Techniques and New Products. The objectives of this Review are to:
- identify, clarify and categorise advances in processes, techniques and technologies that impact on the blood sector and that the NBA should monitor to fulfil its functions under the National Blood Agreement; this should encompass blood collection methodologies, blood donation, donor selection, donation testing, product improvements, new products with widened or new clinical indication, distribution (issue, receipt, storage and transport) and patient blood management
- consider the appropriate active role of the NBA in collecting and analysing this information
- identify developed or emerging technologies and products that may have applicability to the Australian blood sector and their likely impact on future supply capacity, clinical demand and government funding options in the short, medium and long term
- suggest a framework for determining the situations where the NBA might act as a proponent for the new products and technologies
- suggest improved ongoing arrangements for how the NBA can continue to fulfil its role in this area, including any additional consultation arrangements with the sector which may be seen as desirable.
Figure 5.1 The scope of the Review of New Technologies, New Techniques and New Products
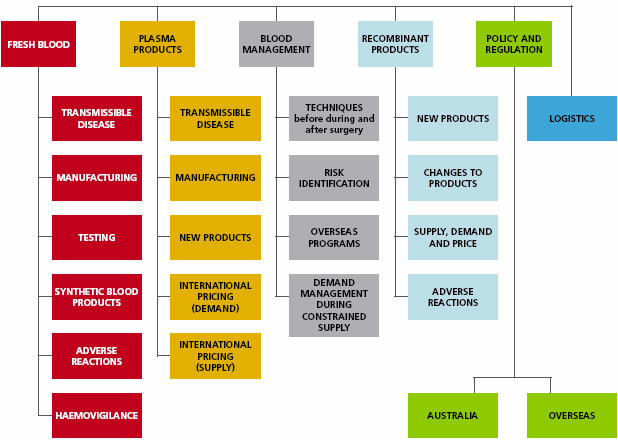
Some of the matters of current interest with respect to fresh blood and plasma products, that will be covered by the Review include:
- known infectious diseases such as Dengue fever, malaria and Chagas’ disease where virus mutation, climate change, travel or immigration may change their expected occurrence in Australia; this would lead to donor deferral to maintain the safety of the blood supply and would have an unfavourable impact on collection volumes
- disease risks such as a possible avian influenza (bird flu) pandemic
- pathogen testing and inactivation; for instance, there is a system operational in Europe which aims to reduce transfusion risks from viruses, bacteria and parasites from platelets
- the continuing development of prion filters to eliminate the risk of variant Creutzfeldt-Jakob disease (vCJD — the human form of mad cow disease)
- the possibility that the emphasis in bacterial testing for transfusion could shift from ‘hold and release’ to ‘bedside’ testing; this would mean the product transfused would be safe at the time of transfusion, not at the time of release
- the impact of apheresis and leucodepletion (potentially increasing plasma collection volumes and increasing safety of whole blood, red cells and platelets)
- the debate concerning transfusion-related acute lung injury (TRALI) and if it is of sufficient risk to suggest that high plasma volume components from female donors should be used for further manufacturing instead of transfusion
- the development of knowledge where transfusion brings relatively assured benefits in excess of potential harm; this follows recent research that suggests transfusion does not always improve outcomes but can cause death or lengthen hospital stays
- the potentially dramatic impact of demand for intravenous immunoglobulin (IVIg) on price if the range of uses for IVIg continues to expand, particularly with positive results being reported from early studies on its use in treating Alzheimer’s disease
- the need for greater consideration of the evidence base for giving transfusions against the principle of ‘first do no harm’
- the continuing expansion in the plasma collection industry in expectation of this increasing demand for IVIg
- the development of new blood typing tests to improve speed and accuracy of typing, eg the United States of America’s (USA) Food and Drug Administration (FDA) announced approval in January 2008 of a system of 14 new tests; the system uses monoclonal antibodies to test for A, B, O, Rh factor and other factors that signify a rarer blood type
- the suggestion that albumin may be used to improve cognitive score in Alzheimer’s disease, thus reducing the potential emphasis on IVIg
- the potential for continuing improvement in outcomes through patient blood management, particularly perioperative management of anaemia, through:
- preoperative treatment of anaemia through dietary supplements or drugs
- cell salvage during surgery
- effective use of coagulation products and tissue sealants
- changes in clinical practice, such as questioning by doctors as to whether transfusion is an automatic consequence of surgery or whether anaemia management is possible by other means
- the development and use of blood substitutes which can act as oxygen carriers, and their trials for efficacy and safety; there are a number of these, some encouraged by battlefield potential, but there also suggestions that their use can result in positive harm.
In the market for recombinant products, matters of current interest include:
- the development of products with:
- longer half-lives
- less demanding temperature conditions for storage
- a wider or more convenient range of vial sizes
- simpler delivery mechanisms
- improved formulation
- ongoing research which may lead to a new product in the monopolistic recombinant factor VIIa market, effectively limiting price increases and improving security of supply
- the possibility for development of recombinant products which could mimic any effect of IVIg in the treatment of Alzheimer’s disease, thus limiting the price increase which would be brought on by increasing demand for IVIg in the face of restricted supply.
Matters of more general recent interest include:
- global developments with respect to stem cell research and use of cord blood
- reported changes in the operation of the USA FDA such as:
- the authority to require postmarketing studies and changes to the labels of approved products
- an increased commitment to overseas inspections, following the widespread contamination of the world’s heparin supply
- the practice of requiring drug makers to study psychiatric side effects in clinical trials
- the ‘Sentinel’ initiative, a strategy to create and implement a national integrated electronic system for monitoring medical product safety
- amendments to its regulations on acceptance of foreign clinical studies that are not conducted under an investigational new drug application; the updated standards are to protect human subjects and ensure the quality and integrity of data obtained from these studies.
The Review of New Technologies, New Techniques and New Products will report formally in December 2008, for consideration by the Jurisdictional Blood Committee (JBC) and the NBA Board. The review process itself, and the interaction with stakeholders concerning their view of future developments, has already had an impact and this will continue as consultations are widened.
It is acknowledged that the need to evaluate current and future developments within and around the blood sector will be ongoing and, as such, continue as a focus in the NBA’s discussions with the regulator, suppliers of blood and blood-related products and the clinical community.
< Previous | Contents | Next >
