2.3 The blood supply chain
Australia’s blood supply consists of fresh, plasma-derived and recombinant products that are vital in the treatment and management of a diverse range of clinical conditions affecting thousands of Australians every day. Australia’s blood sector is funded jointly by the Australian and state and territory governments, with contributions of 63% and 37%, respectively. In 2007–08, governments provided the NBA with $720.6 million to procure and manage Australia’s blood supply (see Table 2.2). Since the establishment of the NBA, governments have spent $2934.7 million on blood and blood-related products.
Table 2.2 Government provided funding to procure and manage the blood supply
| Year | Amount ($ million) |
Growth (%) |
| 2003–04 | 460.5 | – |
| 2004–05 | 536.8 | 16.6% |
| 2005–06 | 577.4 | 7.6% |
| 2006–07 | 639.4 | 10.8% |
| 2007–08 | 720.6 | 12.7% |
| Total | 2934.7 | (average) 11.9% |
The following pages outline the roles and responsibilities of the key stakeholders involved in the Australian blood supply chain as set out in Figure 2.1.
Australian, state and territory governments
As signatories to the National Blood Agreement, all governments are responsible for:
- establishing the policy framework and specific policies relating to the national blood supply
- overseeing the NBA’s management of the blood supply arrangement
- fostering the development and implementation of best practice systems to promote efficient use and minimal wastage of blood and blood-related products
- providing information on demand for blood and blood-related products
- managing local issues.
Therapeutic Goods Administration
The Therapeutic Goods Administration (TGA) is the regulator for blood and blood-related products in Australia. The TGA is responsible for:
- regulating the sector in terms of the efficacy, safety and quality of blood and blood-related products under the Therapeutic Goods Act 1989
- auditing of good manufacturing practice
- product recalls
- modifying safety standards
- issuing directives such as donor deferral.
Figure 2.1 Australian blood supply chain
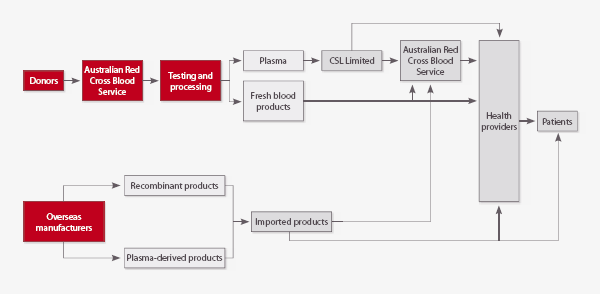
Suppliers of blood and blood-related products
The NBA contracts with a number of suppliers of blood and blood-related products, including:
- the Australian Red Cross Blood Service (ARCBS), for the collection of red cells, platelets and plasma from donors; production, testing and distribution of fresh and some manufactured products; and the provision of donated plasma to CSL Ltd
- CSL Ltd, for fractionating plasma supplied by the ARCBS, and supplying a range of products
- other pharmaceutical companies that are responsible for the supply and some distribution of a range of imported blood products; either the product is not produced in Australia or domestic production capacity cannot meet demand.
Contracts with suppliers for the provision of blood and blood-related products under standing offer arrangements include:
- CSL Ltd, DiaMed Australia Pty Ltd, Ortho-Clinical Diagnostics Inc (USA) and Australian Laboratory Services Pty Ltd for the provision of diagnostic reagents
- CSL Ltd and Octapharma Australia Pty Ltd for the provision of overseas-sourced IVIg
- Baxter Healthcare Pty Ltd, Wyeth Australia Pty Ltd and Novo Nordisk Pharmaceuticals Pty Ltd for the provision of a range of defined blood products.
A standing offer is not a contract but a continuing offer by a supplier or suppliers to provide specified goods and services for a predetermined length of time, usually at a predetermined price. No contract is made until an order is placed which invokes the terms and conditions of the standing offer.
< Previous | Contents | Next >
