National Blood Authority Website
Part Five: Performance: Supporting Appropriate and Safe Use of Blood and Blood Products
National Blood Authority Australia
Annual Report 2010–11
Part 5: Performance: Supporting Appropriate and Safe Use of Bloof and Blood Products
Introduction
The third activity of the NBA's program is to contribute to promoting the safe, high quality management and use of blood and blood products and services. By working with stakeholders and other experts, clinical practices, product use guidelines and quality improvement initiatives are developed that support effective and appropriate clinical practice.
The ultimate objective of our activities is to ensure compliance with best practice in all aspects of the blood sector, achieved by working at a range of levels within an overall framework (see Figure 5.1 below). Information on some of our programs are contained in Part 4-on sector improvement-for example, our new initiative to develop a research program to inform blood sector policy (see page 75). Part 5 focuses on activities which more specifically support enhancements to clinical practice.
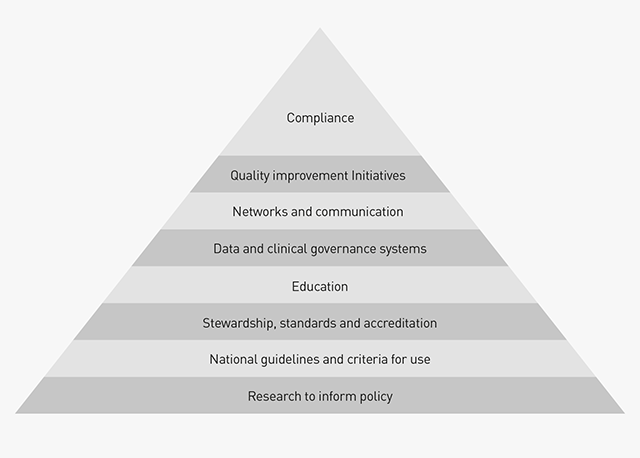
The deliverables and key performance indicators for this function, and our performance, are described below:
Deliverables
| Qualitative deliverables | 2011-12 reference point or target | Results | |
|---|---|---|---|
| Second edition of the Criteria for the use of intravenous immunoglobulin in Australia (the Criteria) is approved by ministers and a communication plan developed to inform the clinical community of its availability | Second edition of the Criteria submitted to ministers for approval by June 2012, along with a communications plan | Met. Health ministers approved the second edition of the Criteria on 20 June 2012.
(see page 90) |
|
| A report on the use of red blood cells in public hospitals is produced | A report on the use of red blood cells in public hospitals is produced by June 2012 | Not met due to delays in availability of jurisdictional data. This report will now be published during 2012-13.
(see page 93) |
|
| Quantitative deliverable | 2011-12 budget | 2011-12 actual | |
| Number of National Health and Medical Research Council Clinical practice guidelines for patient blood management published | 2 | Perioperative module
released in March 2012.
Two further modules will be approved by the end of 2012.
(see pages 88-89) |
Key performance indicators
| Quantitative deliverable | 2011-12 reference point or target | Results | |
|---|---|---|---|
| Quality advice provided to guide promotion of safe, high quality patient blood management and use of blood and blood related products | High level of satisfaction of all funding jurisdictions with the NBA's advice on the promotion of patient blood management (PBM) and use of blood and blood related products as assessed through survey of Jurisdictional Blood Committee members | 89% of jurisdictions were very satisfied with the NBA's work around patient blood management. The remaining 11% were unsure, pointing out that the processes to implement PBM activities
need to take into account varying local health systems, and also the impact of
national health reforms.
(see page 87-89, 95 and 96) |
|
| Qualitative indicator | 2011-12 budget | 2011–12 actuals | |
| Number of hard copies distributed and electronic downloads of guidelines and criteria made available by the NBA | 200 | GUIDELINES: Critical bleeding Module: Copies downloaded-5088 Hard copies-1766 Quick reference guide: Copies downloaded-3250 Hard copies-3326 Perioperative Module: Copies downloaded-3275 Hard copies-2366 Quick reference guide: Copies downloaded-1085 Hard copies-4305 CRITERIA (clinical use of IVIg): Criteria: Copies downloaded-8446 Hard copies-312 Quick reference guide: Copies downloaded-3247 Hard copies-368 (see pages 87-90) |
|
| Number of states and territories contributing haemovigilance data to the national report | 7 | 7 (see page 93-94) |
|
| Proportion of states and territories satisfied with the implementation of the patient blood management guidelines | 100% | 89%, with 11% unsure (see response for the qualitative indicator on the patient blood management guidelines). | |
| Number of colleges and societies that agree to endorse the patient blood management modules | 8 | 8 |