National Blood Authority Australia
Annual Report 2010–11
PART 1: OVERVIEW
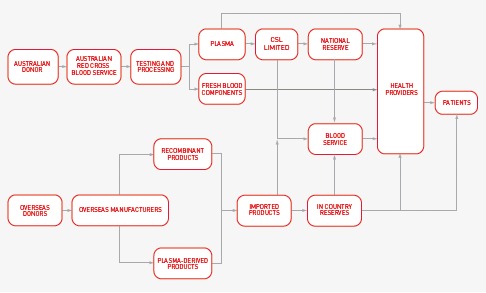
1.3 THE AUSTRALIAN BLOOD SUPPLY CHAIN
The NBA manages the national planning and purchasing of blood and blood products in close cooperation with a number of stakeholders. A summary of the blood supply chain is given in Figure 1.2 below.
FIGURE 1.2 The Australian blood and blood products supply chain
The stakeholders include:
- the Australian Government and state and territory governments as signatories to the National Blood Agreement
- the Therapeutic Goods Administration (TGA) as the regulator for blood and blood products in Australia
- suppliers of blood and blood products: the Australian Red Cross Blood Service (the Blood Service), CSL Limited, Octapharma Australia Pty Ltd, Lateral Diagnostics Pty Ltd (previously Lateral Grifols Pty Ltd), Baxter Healthcare Pty Ltd, Pfizer Australia Pty Ltd, Novo Nordisk Pharmaceuticals Pty Ltd, and OCD (Johnson & Johnson Medical Pty Ltd trading as Ortho-Clinical Diagnostics) and ALS-Abacus.
For further information about our stakeholders, see Part 3: Performance and Appendices 4, 5and 6.
In addition to the networks needed to maintain supply both internationally and domestically, the NBA has developed an effective network of clinical blood sector experts to facilitate the flow and exchange of information in the use of products.
The experience and expertise of clinicians, transfusion nurses and academics in states and territories is essential to optimise the quality use of the products purchased and to identify how current practice can be improved to maximise patient outcomes. This expertise is captured through a wide range of working groups focused on policy and management processes and on evaluating clinical evidence and preparing training material.
Clinical-based committees that assisted the NBA during 2010–11
Anaemia Management Working Group
Australian Bleeding Disorders Registry Steering Committee
Australian Haemophilia Centre Directors’ Organisation (external)
BloodNet User Reference Group
Complex Patient Advisory Group
Haemovigilance Advisory Committee
Imported IVIg Tender Evaluation Committee
Imported Plasma and Recombinant Products Tender Evaluation Committee
National IVIg Criteria Review Working Group
National Patient Blood Management Program Steering Committee
Patient Blood Management Guidelines Steering Committee
Patient Blood Management Guidelines Expert Working Group
Patient Blood Management Guidelines Clinical/Consumer Reference Group
—Critical Bleeding/Massive Transfusion Module
Patient Blood Management Guidelines Clinical/Consumer Reference Group
—Critical Care Module
Patient Blood Management Guidelines Clinical/Consumer Reference Group
—Medical Module
Patient Blood Management Guidelines Clinical/Consumer Reference Group
—Perioperative Module
Red Cell Diagnostic Products Tender Evaluation Committee
Transfusion Medicine Services—JBC sub-committee