National Blood Authority Australia
Annual Report 2010–11
Part 1: OVERVIEW
Blood Products And How They Are Used
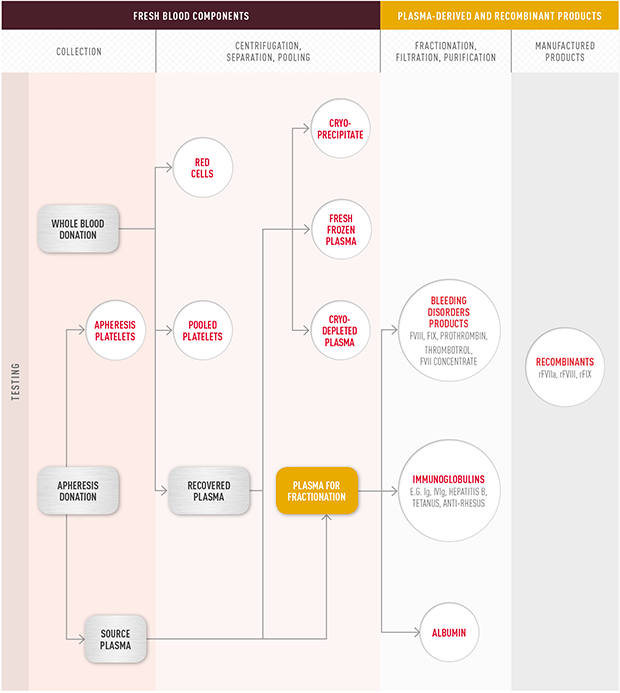
Fresh blood contains red blood cells, white cells and platelets suspended in a straw coloured liquid known as plasma. A blood donor can provide a whole blood donation, or a plasma or platelet only donation through a process known as apheresis. Figure 1.2 The blood product family, illustrates how the various blood products are manufactured.
While whole blood transfusions are still used in certain circumstances, it is a more generally accepted practice to administer the separated, concentrated components of blood. Processing blood into components provides tailored treatment for patients and maximises the use of blood donations.
Fresh blood components-red cells, platelets and fresh frozen plasma (FFP)-are used in the treatment of medical conditions such as cancer, heart, stomach, bowel, liver and kidney diseases. Fresh blood components are also used during and after surgery and to treat people who suffer traumatic injury or burns.
Proteins, isolated by fractionation processes, are made into products to treat specific diseases. For example, clotting factors such as Factor VIII and Factor IX are used to treat haemophilia A and B respectively. Immunoglobulins (Ig) are used by the body to protect itself against infections. Intravenous (IV) delivery of immunoglobulin-IVIg-is used to replace and/or modulate a person's immune response in a wide range of conditions, such as primary immunodeficiency and chronic inflammatory demyelinating polyneuropathy. For some conditions, patients may be dependent on it for their well-being, needing treatment throughout their lives.
Many blood products are made from the plasma component of blood. Plasma contains a large number of proteins each of which performs a different role within the blood. Since the 1940s, it has been possible to extract different proteins from plasma on a large scale. This is commonly referred to as plasma fractionation. In Australia, CSL Limited carries out these fractionation processes on plasma collected by the Australian Red Cross Blood Service (the Blood Service). The volume of plasma to be collected is determined annually by governments and provided under NBA contract arrangements free of charge to CSL Limited to produce the specific blood products needed.
Some blood products are manufactured from non-human components using genetic engineering. These are called recombinant products and are alternatives to some fractionated plasma products. For example, recombinant clotting factors are increasingly used in place of plasma-derived clotting products to treat people with haemophilia.