National Blood Authority Australia
Annual Report 2010–11
Part 1: OVERVIEW
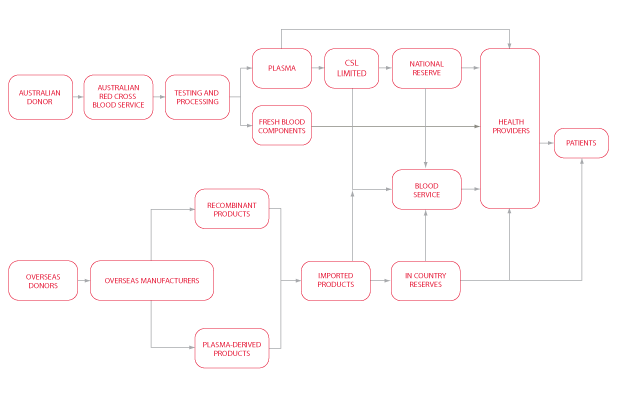
The Australian Blood Supply Chain
The NBA manages the national planning and purchasing of blood and blood products in close cooperation with a number of stakeholders. A summary of the blood supply chain is given in Figure 1.3 below.
Our stakeholders
Blood sector stakeholders include:
- the Australian Government and state and territory governments as signatories to the National Blood Agreement
- the Therapeutic Goods Administration (TGA) as the regulator for blood and blood products in Australia
- suppliers of blood and blood products and diagnostic reagents: the Blood Service, CSL Limited, Octapharma Pty Ltd, Baxter Healthcare Pty Ltd, Grifols Australia Pty Ltd, Pfizer Australia Pty Ltd, Novo Nordisk Pharmaceuticals, Bayer Australia Ltd, Johnson & Johnson Medical Pty Ltd (trading as Ortho-Clinical Diagnostics), Abacus ALS Pty Ltd and Bio-Rad Laboratories Pty Ltd
- health providers and clinicians
- the Australian public, particularly donors and patients.s
For further information about our stakeholders, see Part 3: Performance: Securing the supply of blood and blood products, and Appendices 1 and 4.
In addition to the networks needed to maintain supply both internationally and domestically, the NBA has developed an effective network of clinical blood sector experts to facilitate the flow and exchange of information in the use of products.
Without access to the advice and expertise of our stakeholders, clinicians, suppliers and blood sector managers, we could not achieve the high quality outputs that is expected of the NBA.
Committees and working groups that assisted the NBA during 2011-12
Advisory Group for the Review of the Authorisation and Clinical Governance Framework for Intravenous Immunoglobulin
Anaemia Management Working Group
Australian Bleeding Disorders Registry Steering Committee
Australian Haemophilia Centre Directors' Organisation (external)
BloodNet User Reference Group
Complex Patient Advisory Group
Haemovigilance Advisory Committee
Imported IVIg Tender Evaluation Committee
National IVIg Criteria Review Working Group
Patient Blood Management Steering Committee
Patient Blood Management Guidelines Steering Committee
Patient Blood Management Guidelines Expert Working Group
Patient Blood Management Guidelines Clinical/Consumer Reference Group-Critical Bleeding/Massive Transfusion Module
Patient Blood Management Guidelines Clinical/Consumer Reference Group-Critical Care Module
Patient Blood Management Guidelines Clinical/Consumer Reference Group-Medical Module
Patient Blood Management Guidelines Clinical/Consumer Reference Group-Perioperative Module
FVIII Transition Advisory Group
Schedule 4 and Multi-criteria Analysis/Health Technology Assessment Working Group
Telling health service providers about the NBA
In November 2011 an NBA booth at Australia's largest blood sector conference was a huge hit, attracting up to 970 visitors out of the 1500 delegates who attended the HAA* annual scientific meeting in Sydney.
The booth was located in the exhibition hall at the Sydney Convention and Exhibition Centre in Darling Harbour to promote the role that the NBA plays in the Australian blood sector. The NBA booth was very popular-it was often the busiest booth in the exhibition hall. Visitors to the booth included clinicians, nurses and hospital laboratory scientists. Their average stay was ten minutes, indicating a strong demand for in-depth conversations with NBA staff.
Two NBA projects-Patient Blood Management Guidelines and the NBA's online blood ordering and inventory management system, BloodNet-were the focus. Most delegates were interested in the guidelines-80 per cent of visitors were keen to hear more about the development and roll out of the guidelines and to take a brochure listing all six modules under development by the NBA.
The promotion of BloodNet was supported by live demonstrations of the system to new laboratories that were otherwise unaware of the system. The NBA was able to directly engage scientists from around the country, including more than 40 pathology sites that were not yet using it-especially in Victoria and New South Wales.
Following the success of this booth, the NBA is planning to attend the next HAA conference planned for Melbourne in October 2012.