

In 2008–09 the NBA’s three key strategic directions were to manage and implement governments’ blood sector policy, planning, funding and risk management, to ensure the supply of all required blood and blood products through effective supply contracts with capable suppliers, and to effectively engage with the clinical community in monitoring and improving the appropriate and safe use of blood and blood products to improve patient outcomes.
Effective performance for this strategic objective, as identified in the Portfolio Budget Statements, involves the NBA in developing the National Supply Plan and Budget, ensuring awareness and appropriate management of risks to the supply of blood products, and identifying and implementing a range of activities to drive performance. Having an adequate and secure blood supply are core objectives of the National Blood Agreement, noting that supply shortages of plasma, recombinant and fresh products have been a feature of the global industry for many years. NBA activities during 2008–09 contributing to these objectives are detailed in the sections that follow.
The NBA plays the key role in coordinating both the annual National Product and Services List, and the National Supply Plan and Budget for approval by health ministers. It:
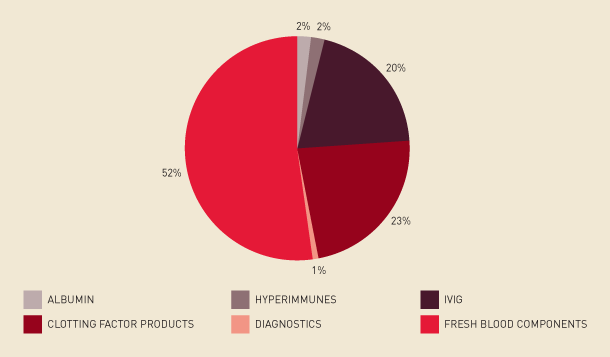
The National Supply Budget for 2008–09 for the supply of blood and blood products and related services was $804.4 million as approved by the Australian Health Ministers Conference. The actual demand for 2008–09 for all jurisdictions amounted to $806.8 million, representing. an $87.3 million increase on 2007–08. Figure 2.1 shows the allocation of this funding to each product category. Products the NBA purchased from suppliers to meet this demand are shown in Table 2.3.
| Supplier | Products purchased | 2007–08 ($m) | 2008–09 ($m) |
|---|---|---|---|
CSL Ltd |
Plasma products
Diagnostic reagent products
Overseas blood products
|
159.90 | 162.09 |
Australian Red Cross Blood Service |
Fresh blood components
|
385.03 | 432.62 |
Baxter Healthcare Pty Ltd |
Overseas blood products
|
80.09 | 84.09 |
Wyeth Australia Pty Ltd |
Overseas blood products
|
42.38 | 48.65 |
Novo Nordisk Pharmaceuticals Pty Ltd |
Overseas blood products
|
17.43 | 17.40 |
Octapharma Australia Pty Ltd |
Overseas blood products
|
34.26 | 46.90 |
DiaMed Australia Pty Ltd |
Diagnostic reagent products
|
0.94 | 0.92 |
Ortho-Clinical Diagnostics |
Diagnostic reagent products
|
0.37 | 0.47 |
Australian Laboratory Services Pty Ltd |
Diagnostic reagent products
|
0.05 | 0.04 |
Total |
720.45 | 793.18 |
Note: Totals do not include interest or the National Managed Fund.
FIGURE 2.1 Funding by product category, 2008–09
Blood expenditure continues to grow at a high rate. The growth in recent years is explained by three major factors:
These factors are best illustrated by analysing the three main elements of growth in blood expenditure in the past six years.
Despite increasing expenditure on plasma and recombinant products, the effect of an overarching commitment to ‘value for money’ can be seen in Figure 2.2, which shows that there has not been any real increase in prices paid for these products since the inception of the NBA. The increased expenditure has arisen from:
The magnitude of the annual savings achieved by current commercial contracts, compared with indexed costs of arrangements before the establishment of the NBA, is also shown in Figure 2.2. In 2008–09 this equated to $39.8 million. The NBA continues to achieve excellent outcomes in price containment, through well-informed and commercially attuned procurement and negotiations.
Achieving further savings on imported plasma and recombinant product prices is unlikely as Australia now pays prices that are competitive in global terms. Any future opportunities to improve affordability are likely to arise from policy and program changes.
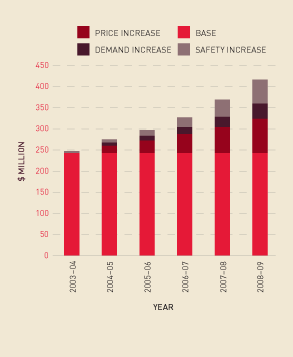
The NBA has limited opportunity to influence the costs of fresh blood components. In the six years to 2008–09 funding for fresh blood and plasma collections has increased from $243 million to $417 million. Of this, $82 million is due to price increases, which have averaged 5.2 per cent a year. Increased demand for fresh products—principally red cells and platelets—has been running at 3.3 per cent a year, resulting in additional expenditure of $36 million. A further $56 million is a consequence of the introduction of government-approved safety measures such as universal leucodepletion of platelets and red cells and bacterial testing for platelets. These safety measures have resulted in an additional increase in expenditure averaging 4.3 per cent a year. The combined effect of these measures can be seen in Figure 2.3.
Steps taken by the NBA, governments and the Australian Red Cross Blood Service in the past two years, such as the KPMG business study, will further support the achievement of business efficiencies within the ARCBS.
The NBA and the Jurisdictional Blood Committee are supporting initiatives to minimise demand increases by exploring the scope to reduce wastage and increase the use of products in accordance with best-practice standards. In 2008–09 the NBA continued a range of activities to gain commitment to these objectives from, and to engage, pathology services, hospitals and clinicians.
FIGURE 2.2 Plasma-derived and imported product expenditure, 2003–04 to 2008–09
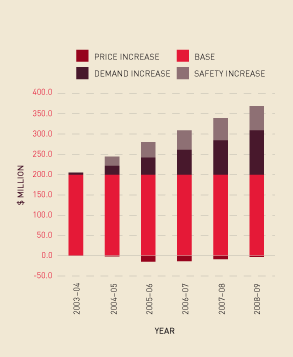
Note: Increases are cumulative, with 2003–04 as the base year.
FIGURE 2.3 Fresh blood expenditure, 2003–04 to 2008–09
The NBA administered budget, which is used principally to purchase blood and blood products, is developed annually as part of the National Supply Plan and Budget (NSPB) process agreed by the Australian Health Ministers Conference. In consultation with the jurisdictions, the NBA reviews the National Supply Plan and Budget as part of the mid-year review process, to adjust jurisdictional funding requirements as a result of the demand seen in the preceding six months.
The end-of-year result against the NSPB approved by the Australian Health Ministers Conference varied by only 0.4 per cent, despite higher than expected growth seen for most clotting factor products during the first six months. This resulted in a mid-year increase of $24.8m as shown in Figure 2.4 and Table 2.4. However, in the second six months the growth rates for these clotting factor products slowed considerably, so the anticipated increased need for funds did not eventuate. The NBA continues to be hampered in its ability to explain changes in demand by the lack of data. Full implementation of the Australian Bleeding Disorders Registry should improve our capabilities in this area in future years.
FIGURE 2.4 Actual issues performance against the National Supply Plan and Budget and the mid-year review, 2006–07 to 2008–09.
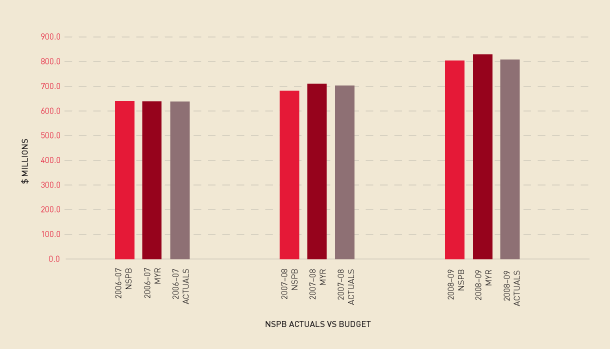
Note: ‘NSPB’ denotes National Supply Plan and Budget; ‘MYR’ denotes mid-year review.
| 2007-08 Actual $ Issued |
2008-09 NSPB $ |
2008-09 Mid Year Review $ |
2008-09 Actual $ Issued |
|
|---|---|---|---|---|
| Albumin | 13,485,878 | 15,202,965 | 14,862,753 | 14,809,333 |
| Total IVIg | 133,464,493 | 159,213,886 | 157,627,222 | 155,682,634 |
| Total Factor VIII | 98,011,446 | 106,340,959 | 112,901,071 | 104,155,865 |
| Total Factor IX | 22,170,203 | 22,638,609 | 27,636,278 | 26,064,039 |
| Factor VIIa | 17,432,671 | 17,770,894 | 20,613,661 | 17,403,879 |
| FEIBA | 11,952,350 | 10,222,500 | 17,472,500 | 13,476,300 |
| 296,517,041 | 331,389,813 | 351,113,485 | 331,592,049 |
The NBA has always given high priority to our obligation to manage blood sector risks, especially those related to supply security. We do this by ensuring that responsibility and accountability lie with those best placed to manage risk. During 2008–09, this required the NBA to:
All supply contracts have a requirement for the suppliers to develop and provide risk-management plans to the NBA. These plans detail the supplier’s approach to ensuring that risks in relation to the provision of products and services are identified and avoided or mitigated as far as possible.
The National Blood Supply Contingency Plan was officially launched by Senator the Hon Jan McLucas, then Parliamentary Secretary for Health and Ageing, at the National Blood Sector Conference held on 6 and 7 November 2008.
The Plan consists of two parts:

NBA staff preparing to mail out the Plan.
The Plan was activated for the first time on 22 August 2008 and remained at ‘White Alert’ until 23 October 2008 in the light of a continued tight supply of red cells.
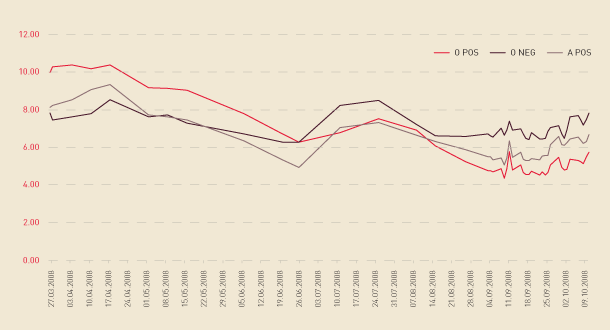
Figure 2.5 shows the decline in stocks of red cells, beginning in April with A positive stocks. This then resulted in a more rapid decrease in O negative stocks than anticipated, which, when combined with the usual reduction in stock due to seasonal flu and a long tail to the flu season, put sustained pressure on red cell stocks. This supply situation necessitated weekly teleconferences to monitor stock levels and trends and to engage treating clinicians in maintaining a normal operating environment despite reduced inventories. During the reporting year the combined red cell inventory of the Australian Red Cross Blood Service and Australian health providers fell below the minimum stock level of five days for a total of eight days. Effective monitoring of stocks at the time and continual communication with health care providers by jurisdictions and the ARCBS meant that no further action was required.
A number of improvements to the National Blood Supply Contingency Plan have been agreed as a result of this, the first activation of the plan. These include the development of an intensive product management protocol for red cells to ensure that early warning signs of a risk to supply are acted on in a timely manner, thereby reducing the need to activate the plan.
FIGURE 2.5 Days of red cell supply, by blood type, March to October 2008
In response to the Victorian bushfires and the Ashmore Reef incident, the NBA worked collaboratively with the Australian Red Cross Blood Service to provide regular information to governments on stocks, ensuring the movement of stock to where need was greatest. While the jurisdictions and the NBA were very active in monitoring demand and participating in whole-of-government processes when necessary, the actual requirement for additional blood products was low and easily managed within existing inventories.
The tragedy of the bushfires demonstrated the generosity of the Australian public in donating blood at times of crisis. Figure 2.6 shows the dramatic increases in fresh blood donations at the time of the Bali bombing and after the Victorian bushfires. The number of new donors in February 2009 was the highest ever recorded for the Australian Red Cross Blood Service.
FIGURE 2.6 Donor attendances in response to a disaster
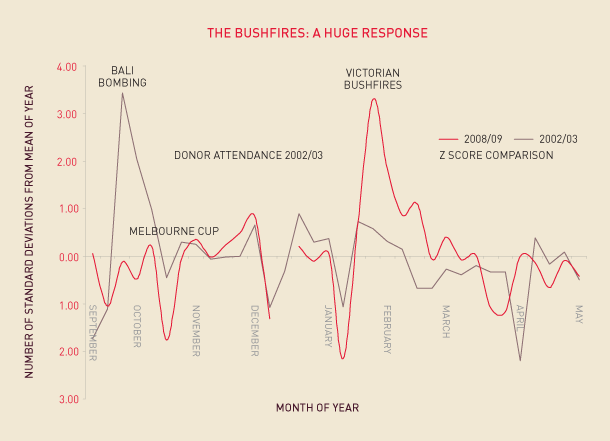
Notes: Figure is a Z score comparison.
Source: Australian Red Cross Blood Service.
In response to the outbreak of pandemic (H1N1) 2009 virus, the NBA closely monitored stocks and the evolving international analysis. The increased rate of infection by the virus has the potential to have two major impacts on the blood supply:
The NBA has worked closely with the ARCBS to monitor the situation and to ensure that planning is appropriate. Current activities include modelling the possible impact of the pandemic (H1N1) 2009 virus on inventory and refining strategies to target donors of the most essential types of blood components, such as platelets and O negative red cells, should these be required.
Following action by the NBA, the ARCBS was the first international blood service to announce a response to the pandemic (H1N1) 2009 virus. This included:
As the rate of infection continued to grow, the NBA increased the frequency of its communication with the ARCBS to confirm that appropriate response strategies were being planned. ARCBS activities included:
The NBA will continue to provide to all governments regular reports on stock levels and timely advice on inventory trends. If these trends indicate a risk to the blood supply, the NBA will activate the National Blood Supply Contingency Plan to ensure that available stocks are managed in the most efficient manner. The impact of the pandemic (H1N1) 2009 outbreak on blood supplies will be a continuing issue in 2009–10.
The NBA has a number of projects designed to improve the overall efficiency of the sector as a key element of improving affordability and risk minimisation. These projects require detailed research and are always implemented with the continual input and guidance of stakeholders and experts in the field of inquiry. During 2008–09 these activities involved the NBA in:
Under the National Blood Agreement, interested parties can make proposals for changes to products or services on the National Product and Services List. Schedule 4 of the Agreement provides for evidence-based evaluation, information and advice to support decisions on these changes in the context of the primary and secondary objectives of the Agreement. To date, the Jurisdictional Blood Committee, on the NBA’s advice, has been effective in assessing new products when they are similar to, and hence can be directly compared with, existing products, and there are no new policy considerations. The procedures are detailed in a document entitled Information and guidance for applicants on submitting a national blood supply change proposal, available at www.nba.gov.au.
In 2008–09 the NBA commenced development of a comprehensive framework to support complex assessment of products in the context of the objectives of the National Blood Agreement. Typically, these products raise complex policy considerations or the cost-effectiveness evaluation has complex impacts beyond the blood sector. The NBA’s framework has 13 criteria:
| 1. | Security of supply |
| 2. | Comparative health gain |
| 3. | Comparative safety gain |
| 4. | Comparative cost-effectiveness |
| 5a. | Financial implications for the national blood budget |
| 5b. | Financial implications for government health budgets |
| 6a. | Self-sufficiency—reliance on domestic production |
| 6b. | Self-sufficiency—efficiency of domestic production |
| 7. | Donations |
| 8. | Accessibility and utility |
| 9. | Feasibility |
| 10. | Clinical need |
| 11. | International practice |
This framework:
The evaluation process clearly defines the assessment criteria and an evaluation pathway and is arguably more robust than processes that apply to blood products elsewhere in the world. The framework has been agreed in principle by the Jurisdictional Blood Committee and is currently the subject of consultation.
In 2007 governments endorsed a recommendation in the review of Australia’s plasma fractionation arrangements that the NBA should also review existing arrangements for distributing blood products. This review was to focus on the distribution supply chain for plasma-derived and recombinant products but not include supply chain analysis for fresh blood. Phase 1 of the review was undertaken in 2008–09 and concluded that current arrangements are neither ideal nor optimal from cost and governance perspectives and that there are significant opportunities to rationalise activity. The review identified three potential areas for improvement, and in February 2009 the Jurisdictional Blood Committee agreed to implement Phase 2 of the study. This phase will explore the potential costs and benefits of the changes required to achieve these improvements.
The Statement of Expectations project, previously described as the Agreement to Supply project, has arisen because of concern that there are no specific accountability obligations on laboratories or other institutions that receive blood products for dispensing to patients. These facilities are regulated for safety and quality by other agencies, but they have no stewardship obligations under the National Blood Agreement, even though they receive the products free of charge. In particular, the NBA expects that, as a key part of the blood supply chain, the facilities will use rare and expensive products in an efficient and respectful manner. The NBA and the Jurisdictional Blood Committee consider that the facilities should be encouraged to undertake appropriate inventory management and reporting, implement appropriate ordering and receipt verification, encourage adherence to relevant guidelines, standards and legislation, and provide data, including adverse event data. Following stakeholder consultation, the NBA has reconsidered the best way forward on this project and will be submitting its proposal to government in early 2009–10.
Implementation of systems and processes that provide accurate and timely data is essential to ensure quality and informed decisions on the use of and demand for blood and blood products and services. During 2008–09 the NBA progressed activities in the areas detailed in the following paragraphs.
The redeveloped Australian Bleeding Disorders Registry ‘went live’ on 18 December 2008 and was rolled out to all users, representing the culmination of significant work on the part of NBA staff in collaboration with a range of sector stakeholders.
The aim of the ABDR project is to develop and implement a system that provides to all stakeholders information on patients with haemophilia and other bleeding disorders. The NBA and the states and territories have a strong interest in improving understanding of the nature of product demand and use, which will enable more reliable supply forecasting and planning. Use of the ABDR will also enable the high-cost patient pool to be calculated. Implementation of the ABDR, in a multitude of different hospital IT and administration systems, has proved especially daunting.
The NBA is assisting haemophilia treatment centres, which are entering data, in the transition to the new system. Reporting requirements are among the highest priority items outstanding, and these are currently being finalised.
The aim of the Integrated Data Management System is to provide the NBA with an enhanced internal capability for data management. This will improve budgeting and forecasting of supply and demand for blood and blood-related products, inventory management, contract administration, and management of Financial Management and Accountability Act 1997 reporting obligations.
The system became operational at the end of June 2008, but there have been a number of hurdles to overcome before it is fully useable. Several successful enhancement and defect builds were carried out during 2008–09, and a final verification and reconciliation was completed in March 2009. Work on the business intelligence reporting tool is under way, and it is expected that the Integrated Data Management System will become integral to our operations during 2009–10.
The National IVIg Management System was initiated to capture information about the use of intravenous immunoglobulin to ensure alignment with the Criteria for the clinical use of intravenous immunoglobulin (IVIg) in Australia. The system will enable the collection of data on requesting, ordering, authorisation and treatment outcomes for IVIg and will provide accurate and comprehensive information on the use and efficacy of IVIg, which will be reported back to the clinical community. The increasing and competing pressure for access to limited product highlights the need for greater awareness of the efficacy of IVIg for a range of conditions, so that supplies can be directed to areas where the product is likely to be of clinical benefit.
During the development of the technical specifications for the system there has been extensive research and consultation with stakeholders. In addition, there are several existing data capture systems in use that meet some of the user requirements for the new system. In 2008–09 detailed specification documentation was developed, and systems within the broader sector were reviewed to identify opportunities for leveraging from the Australian Red Cross Blood Service’s STARS (Supply Tracking and Reporting System) and Queensland Health’s ORBS (Ordering and Receipting Blood System).
The potential for development of the National IVIg Management System within the ORBS and/or Australian Bleeding Disorders Registry frameworks, and further sector IT integration, is the subject of ongoing work. The NBA will explore options for a more integrated, innovative and broad approach to the overall management framework for blood products in Australia, including IVIg, during 2009–10.