

In 2008–09 there was continued progress on a number of activities designed to move forward with the second major objective of the National Blood Agreement—increased appropriateness of use of blood and blood products.
In 2008–09 the NBA made good progress towards implementing the recommendations of the Initial Australian Haemovigilance Report 2008. Governance arrangements for the enduring National Haemovigilance Program in Australia were endorsed by the Jurisdictional Blood Committee, and funding was allocated to support direct program delivery. National minimum haemovigilance data set definitions were finalised, and governance arrangements for the ongoing Haemovigilance Advisory Committee were developed for consideration by the Committee at its first meeting in July 2009.
To help the states and territories implement the minimum national data set, specifications were developed to enable them to analyse the capability of their health care information systems to provide haemovigilance data. Assessments have been completed in Victoria, Tasmania and the Australian Capital Territory, and all other jurisdictions are due to report on their capability before the end of 2009.
In the mid to long term, the needs of data collection projects would best be met if suitable clinical coding existed. This would simplify obtaining data by unifying the data sources such that all relevant data on blood-related activities resided in hospital systems’ central patient records. Currently, ICD-10-AM codes and AR-DRG codes are not specific enough to directly capture blood-specific data and treatments involving blood data. Neither are the specifications in SNOMED-CT. However, a number of agencies in Australia are working to change and update the ICD and SNOMED codes and standards.
An NBA proposal for a national indicator relating to transfusion complications was included in a final report from the Australian Institute of Health and Welfare, which is recommending indicators for national reporting on safety and quality. The Australian Commission on Safety and Quality in Health Care is considering this report and will forward its recommendations to the Australian Health Ministers Advisory Council.
If Australia can begin to implement definitions for blood management and transfusion-related data, it will lay the groundwork internationally with the World Health Organisation for ICD-11. Australia has a strong international reputation in this field, and many countries have previously adopted Australian coding standards. This would be an excellent opportunity for Australia to again lead the way. The Haemovigilance Advisory Committee is exploring the processes for defining new clinical coding standards for transfusion-related adverse events in the ICD-10-AM codes via the National Centre for Classification in Health. Further work will also be carried out with the SNOMED Clinical Terminology from the National eHealth Transition Authority.
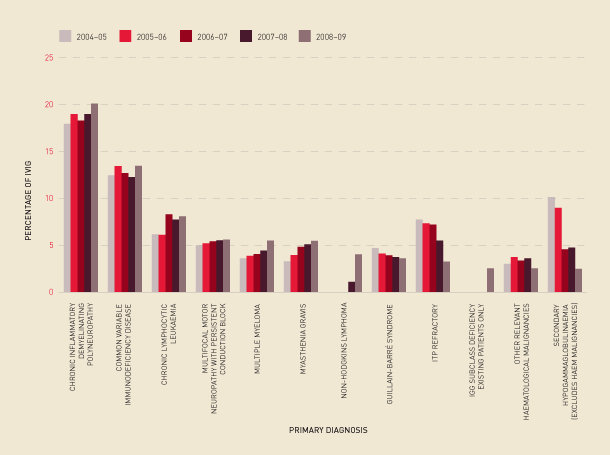
Analysis of use against the conditions within the Criteria indicates an increase of 10.6 per cent in the number of grams issued from 2007–08 to 2008–09, and the top 12 primary diagnoses accounted for 76.6 per cent of issues. The largest increases were found in chronic inflammatory demyelinating polyneuropathy, common variable immunodeficiency disease, multiple myeloma, and myasthenia gravis. Inter-jurisdictional variations (in grams issued per 1000 population) were found in most diagnoses.
The top 12 uses of IVIg and the changes in these uses over time are shown in Figure 2.21.
The NBA established a group, endorsed by the Jurisdictional Blood Committee, to respond to questions arising from implementation of the Criteria. This group includes representatives of IVIg gatekeepers in each state and territory, IVIg discipline sub-groups (formed when the Criteria were developed), the Commonwealth and the JBC. A smaller ‘resolution’ group was also formed, consisting of a clinical representative of the Commonwealth, a JBC representative and the chairs of the IVIg discipline sub-groups.
Seventeen issues have been considered so far, and resolution has been provided on the majority—for example, on ambiguities in the wording of the Criteria.
Issues requesting the addition of an indication to the Criteria fall outside the scope of the process and will be considered in the context of the JBC-endorsed triennial formal review, due to commence in December 2009.
FIGURE 2.21 Top 12 uses of IVIg, 2004–05 to 2008–09
The purpose of the red cell utilisation project is to support the application of a data linkage model in each state and territory that:
All jurisdictions have agreed in principle to implement a data linkage exercise in their state and territory. They have agreed that this should be a national initiative and have identified a number of matters that need to be addressed, including:
The first meeting of the project’s working group occurred in May 2009, and a work plan and an implementation strategy were prepared. Discussion began with the data required and led members to identify an ideal set of data. The feasibility of obtaining the data will be determined by a business analyst, who is to be engaged to support the project.
The 2008 National Blood Sector Conference, ‘Better Blood Management in Australia—what’s stopping us?’ was held at the Coogee Beach Crown Plaza hotel on 6 and 7 November 2008. The theme of the conference was to share, discuss and explore actions that can be taken to remove barriers to the wider adoption of best practice in the blood sector.
The focus of the conference was to provide a forum in which leading clinical and administrative practice in use and management of blood, blood products and their alternatives could be demonstrated and advanced, thereby building sector capacity. The conference attracted strong interest from all parts of the blood sector and provided a focused opportunity to learn about clinical change initiatives both locally and internationally.

The 2008 blood sector conference called for action by the audience to ensure a successful and sustainable future for the sector through exemplary stewardship from all players in careful management of both blood supply and blood demand.
The conference covered the following subjects:
The conference also provided examples of leading clinical practice in cardiothoracic surgery, obstetrics, and intensive care and anaemia management. It attracted almost 170 national and international delegates and generated strong interest in and support for the adoption of a new paradigm in the management of blood. Participants especially enjoyed the lively and entertaining ‘Hypothetical: The blood sector – what to expect in the next 10 years’, facilitated by Doctor Norman Swan, that concluded the conference.
The conference proceedings (presentations and audio recordings) were provided on a CD to all delegates. Copies of the proceedings are available via the NBA website. Since the conference there have been 78 downloads of the proceedings.
The Clinical Practice Guidelines on the use of blood components were published in 2001. At the time some important areas of practice (specifically obstetrics and paediatrics) were omitted from the original publication, and since the guidelines’ publication new clinical and scientific data have emerged. The current review is providing an opportunity to include these areas and also to fill other gaps in coverage—such as critical bleeding scenarios, haemodynamically unstable patients and chronic transfusion recipients. The aim of the review is to develop evidence-based recommendations to guide health practitioners in effectively managing patients who require blood management intervention. This focus is consistent with the draft national safety and quality framework released by the Australian Commission on Safety and Quality in Health Care in early 2009 which advocates a patient centred approach to care.

Jen Roberts (left) from the NBA with NHMRC expert working group co-chairs, Dr Amanda Thompson and Dr Craig French.
The management framework for coordinating the review consists of an overarching steering committee and an expert working group made up of nominees provided by relevant colleges and societies. The expert working group is responsible for clinical oversight of the guidelines—in particular, ensuring that the research undertaken is comprehensive and the quality of the revised guidelines meets with clinical approval. Clinical and consumer reference groups are providing essential specific and expert knowledge to guide the development of the guidelines and advice on the guidelines relevance to and utility for the target audience. All levels of the management framework are assisted by a Guidelines Assessment Register expert appointed by the NHMRC.
The NBA has completed an extensive search for blood-related guidelines developed internationally to inform the expert working group on the scope and possible questions to guide the systematic review. In response, the expert working group has decided to produce a range of guidelines, as follows:
The role of the clinical and consumer reference groups, while important in confirming the scope of the questions, will become ever more important as the research and outcomes of the systematic review become available and final recommendations or consensus statements are developed.
The guidelines will be externally reviewed prior to being considered for endorsement by the NHMRC.
The NBA and the New South Wales Clinical Excellence Commission have co-sponsored an innovative approach to communicating the latest evidence on patient blood management to surgeons. The web-based transfusion debate and information portal http://thetransfusionquestion.com.au was aimed at promoting robust debate between eminent patient blood management practitioners from the international community and Australian surgeons and anaesthetists who prescribe blood components.
Seven debating streams were made available: inappropriate red cell transfusion; dosage (one versus two units); infection and transfusion; the true cost of blood; pre-operative assessment and measures; intra-operative management; and ‘What is patient blood management?’. A group of about 20 international experts contributed to and monitored the debates. In addition, a ‘Research’ section on the website provided details of relevant recent peer-reviewed publications in transfusion and cardiac surgery, anaemia management and red cell transfusion avoidance, transfusion practice in orthopaedics, the cost of blood and transfusion risks.
The site has proved to be popular and well supported, with over 3000 visits from 59 countries; Australia and the United States account for the largest groups. The success of the project will assist in informing future change management strategies in light of the new focus of the National Health and Medical Research Council guidelines.
The website was promoted by the development of posters that challenged common views about transfusions.
In 2006 the South Australian Department of Health funded the development of an online education package for clinical staff involved in the transfusion chain—including medical officers, nurses and midwives, and courier or porter staff that transport blood products. The eLearning tool has been successfully used in South Australia, and other states and territories have expressed considerable interest. During 2008 a total of 10 176 registrations were received, of which 97.7 per cent were from professionals working throughout Australia. The registration rate continues to be strong. The nursing profession is the most prevalent user of the program. Nationally, the completion rate for local participants is about 65 per cent.
During 2008–09 the NBA worked with South Australia Health to develop governance arrangements to enable this tool to become sustainable nationally. A steering committee has been established, with membership from the states and territories, the NBA, the Australian Red Cross Blood Service and the Australian and New Zealand Society of Blood Transfusion. The steering committee developed recommendations for consideration by the JBC at its meeting in May 2009 and a three-year project has now been agreed to expand the use of the tool nationally.
In February 2009 the NBA obtained approval from the JBC to provide funds additional to those already committed by the Victorian Department of Human Services to support the redevelopment of the Graduate Certificate in Transfusion Practice course material for delivery in the 2010 academic year. The course, developed, owned and managed by the Victorian Department of Human Services, is the only formal transfusion-specific tertiary qualification especially intended for transfusion improvement among practitioners working in Australasia. It is available online and has been completed by students from other states and territories.
Redevelopment of the course will enable the inclusion of emerging transfusion patient safety and quality requirements, and alignment with the role of a transfusion nurse or practitioner; it also provides an opportunity for Victoria to incorporate national input into the curriculum’s content. To this end the NBA participated in a national workshop in April 2009. The workshop discussed every aspect of the course and its materials in the context of the evolving roles of transfusion nurses and practitioners, with the express intention of translating national best-practice guidelines into everyday transfusion practice.
The program has now been accredited by the Academic Board of the University of Melbourne, which provides academic input to the program’s development and delivery. This accreditation will result in a co-branded qualification from the Australian Red Cross Blood Service and the Victorian Department of Human Services. The course is administered by the School of Nursing and will continue to be delivered in partnership with the ARCBS under the auspices of the ‘Blood Matters: better, safer transfusion program’ with the objective of increased national uptake.
‘Patient blood management’ is defined as a ‘multidisciplinary team approach to the appropriate use of blood products, utilising techniques that minimise blood loss and avoid unnecessary transfusions by optimising a patient’s anaemia tolerance and red cell mass’1,2.
There is growing interest in and commitment to advancing the principles of appropriate blood use. This was clearly evident at the November 2008 National Blood Sector Conference, at which a number of presentations variously described the Western Australian blood management program, the American experience in blood management, and the importance of anaemia management. Appropriate patient blood management techniques are increasingly being assessed as being better for the patient and—importantly in the current economic environment—for hospitals and state and territory health systems. It is also clear that a whole-of-institution approach is essential to implementing and maintaining an effective blood management system.
During 2008–09 the NBA organised presentations to the Jurisdictional Blood Committee on a number of aspects of patient blood management, and in February 2009 the JBC endorsed a National Patient Blood Management Program. The NBA has begun the first phase of the work program, focusing on the establishment of appropriate governance arrangements to create national leadership, and conducting research into:
The Blood Measures project is a collaboration between the NBA and the ARCBS and addresses the current difficulty in obtaining good quality data on the clinical effectiveness and use of blood components. Although there have been a number of separate initiatives to investigate the use of blood components within Australia, comparison between these studies is problematic due to the differences in data types, definitions and methodologies. The project aims to achieve widespread adoption of standard parameters that are indicators of blood-related use across a range of clinical scenarios.
A Steering Committee chaired by Professor James Isbister commissioned the Department of Epidemiology and Preventive Medicine at Monash University to undertake a desktop review of a range of indicators of blood product use in Australia and internationally. A National Working Group was convened, comprising clinicians in a range of disciplines and other stakeholders such as nurses, transfusion scientists, epidemiologists and hospital administrators.
The National Blood Measures Working Group has now developed a draft national guide containing suggested standard measures and data definitions that can be used in any study of transfusion practice. Where possible, the measures are epidemiologically sound—that is, the data are routinely collected, routinely accessible and can be consistently interpreted.
The draft guide became available on the NBA website in June 2009 in order to obtain further input from blood sector-specific stakeholders. The NBA will work in accordance with the national health data governance framework in finalising the standardised set of blood measures.

The Blood Measures Guide

A sample measure

The aim of the Consultative draft guide to the set of standard indicators for use in measuring fresh blood components in Australia is to provide a tool that can be used by those investigating the use of fresh blood components. It contains a nationally agreed set of standard measures that can be used in any investigation of blood or blood component use. Perhaps surprisingly, this is the first time such a guide has been prepared anywhere in the world.
The Blood Measures project focuses on the patient end of the blood supply chain, providing advice on the collection of reliable, accessible, consistently interpreted and objective data on the use of fresh blood components. An easily accessible set of standard measures that can be used for any data collection project would be useful throughout the health sector, and we hope that this guide will be used by clinicians, nurses, auditors, transfusion scientists and researchers.
The National Working Group developed the guide during 2008–09 and the NBA is grateful for members’ valuable contribution. There was wide-ranging consultation about the importance of measures, their definitions and how they should be expressed.
The resulting draft guide was uploaded onto the NBA website at the end of June 2009. Although use is voluntary, we hope the guide will be widely used and the measures given a thorough trial. Users are encouraged to comment on which measures work well, which need further refinement, which are irrelevant, and which might be missing. The NBA will consider all the feedback in consultation with the National Working Group and then re-publish the guide.