National Blood Authority Australia
Annual Report 2010–11
Part 3: PERFORMANCE
3.3 MANAGEMENT OF RISK AND SECTOR PERFORMANCE IMPROVEMENT
The NBA gives a high priority to managing blood sector risks and contingency planning to ensure that the supply of all required blood and blood products is maintained. A key mechanism to reduce risk that the NBA has focused on during the year is to drive improved data capture and analysis across all aspects of the supply chain.
| Key performance indicators | Measure | Results | |
| Management of the National Blood Supply Contingency Plan (NBSCP) | High level of satisfaction of all funding jurisdictions with the NBA’s management and implementation as assessed through a survey of JBC members | 78% of jurisdictions were very satisfied with the NBA’s advice, noting that the implementation of the BloodNet system will further improve timely inventory management. The remaining 22% were unsure. (Page 58) | |
| Number of days the National Blood Supply Contingency Plan is activated for plasma and recombinant products | 7 24-30 September 2010, as a precautionary measure to guide the transition to new supply arrangements following the Octagam recall. (Page 59) |
||
| Deliverables | Measure | Results | |
| Implement new product levels for national reserve inventories and new supplier contract arrangements | New levels to be implemented by 30 June 2011 | Required product inventory levels implemented as an outcome of the transition phases under the CAFA. New tenders for imported IVIg and other imported plasma and recombinant products include enhanced framework for supply security inventories and updated required product inventory levels. Comprehensive updated supply security risk assessment for plasma and recombinant products now to be conducted in 2011–12. (Pages 47–55, 67) |
|
| Percentage of recommendations from the Administrative Review of the National Blood Arrangements 2009, for which the NBA has responsibility completed¹ | Number of measures from the implementation plan that the NBA was assigned and successfully implemented. >=95% |
3 of 4 (75%) recommendations that the NBA was assigned have been completed. Work on one further recommendation is ongoing together with the JBC and CTEPC. |
1 It is expected that all recommendations will be implemented by 2013-14
RISK MANAGEMENT
The NBA continues to give high priority to our obligation to manage blood sector risks, especially those related to supply security. We do this by ensuring that responsibility and accountability lie with those best placed to manage risk.
A key strategic direction for the NBA in 2010–11 was to ensure the most effective risk management arrangements were in place to implement blood policy and to continue to improve sector performance.
Key activities included:
- continued scrutiny of compliance with, and the quality of, risk management strategies contractually required of suppliers
- development of an overall financial reserve strategy
- management of both real and potential risks to the supply plan during the year
- identification of jurisdictional preparations for managing risk.
Risk management for contracted supply arrangements
All supply contracts have a requirement for the suppliers to develop and provide risk management plans to the NBA. These plans detail each supplier’s approach to ensuring that risks in providing products and services are identified and avoided or mitigated as far as possible. They provide a basis for discussions on risk management with suppliers.
The CAFA, as the contract dealing with domestic fractionation, also establishes a requirement for an annual risk workshop between CSL and the NBA. Suppliers’ risk management plans are taken into account by the NBA in developing its own risk management plans for the contracted supply arrangements for each supply contract or suite of contracts.
In the course of tendering and contracting for blood and blood products the NBA has developed a framework of arrangements relating to supply contracts to provide robust risk management aimed at security of supply. These include:
- notification and reporting processes to identify impending risks
- intensive product management mechanisms
- commitments from suppliers to accord preferred customer status to supply for Australia
- requirements for products to have a specified minimum level of shelf life at the time of supply in Australia
- requirements for the holding of required levels of in-country reserves
- provision for supply of alternative products, if triggered by the NBA
- in some cases, multiple supplier arrangements.
In the tender processes for new contracts for imported IVIg and other imported plasma and recombinant products (see pages 49–53 for more information on these procurements), the NBA has introduced enhanced and additional supply security measures, including:
- a committed global stock requirement for products with a steady demand—a supplier will arrange for a quantity of product held in the normal global supply chain to be specifically earmarked as product available for supply to Australia
- contractual procedures specific to the management of a product recall situation
- alternative minimum inventory requirements to apply for products where low level or highly spasmodic demand for particular products makes the NBA’s normal in-country reserve requirements difficult for suppliers to commit to on a sustainable basis.
While these advances in supply risk management have been made, reprioritisation of resources during the year meant that the planned updating of the NBA’s supply risk analysis for plasma and recombinant products did not progress. This process will be undertaken during 2011–12.
Financial risks
The new contractual arrangements with commercial suppliers and the implementation of the output based funding model introduce some financial risks for the NBA. Accordingly
in the context of developing the 2011–12 NSP&B, the NBA prepared an overall financial reserve strategy, taking into account:
- the completion of risk analysis of plasma and recombinant products and establishment of reserve levels
- an understanding of all potential calls on cash reserves
- a sensitivity analysis of the impact on cash flow of demand changes against the agreed supply plan.
The National Blood Supply Contingency Plan
The National Blood Supply Contingency Plan (NBSCP) was activated during the year due to the voluntary recall of Octagam during September 2010, see page 59, but otherwise inventory levels for all products remained strong.
The NBA liaised closely with suppliers to monitor possible impacts during the Queensland and Victorian floods in January and February 2011 and the Australian-New Zealand air travel interruptions due to volcanic ash in May and June 2011. All suppliers were well prepared
and there were no material impacts on any blood or blood product supply arrangements for Australia.
The NBA also reviewed its Standard Operating Procedures for the NBSCP and these were updated to take into account the lessons learnt from the recall of Octagam. An internal education campaign will be undertaken during 2011 to ensure both current and new NBA staff members are aware of their roles and responsibilities during the activation of the NBSCP.
Due to a reprioritisation of internal resources, the annex covering transfusion-transmitted infection for the NBSCP was not completed during 2010–11, although a draft of the roles and responsibilities was prepared.
Management of risk in states and territories
States and territories have constitutional responsibilities for coordinating and planning the response to disasters within their jurisdiction; in turn their emergency management organisations coordinate with Emergency Management Australia.
The extent to which jurisdictions have prepared responses to risks to the blood supply varies depending on their level of knowledge of the total inventory and clinical requirements and the effectiveness of linkages between the NBSCP and state emergency response plans.
Some jurisdictions have developed formal emergency blood management plans or have specific blood sector elements within wider state emergency plans, while others rely on knowledge of the sector and informal networks. Several states have recently conducted exercises to test their procedures.
During 2010–11, the NBA collected information on the preparedness of jurisdictions for emergencies involving blood, and the JBC will consider these early in 2011–12.
VOLUNTARY RECALL—NBA ENSURES IVIG SUPPLY
The NBA ensured that patients currently receiving intravenous immunoglobulin (IVIg) continued to receive products required for their treatment after Octapharma Australia recalled all batches of its Octagam (IVIg) solutions, due to safety concerns, on 24 September 2010. Octapharma issued the voluntary recall in consultation with the TGA, as a result of an increase in adverse events internationally, although none had been reported in Australia at that time.
On the same day, the NBA activated the National Blood Supply Contingency Plan (http://www.nba.gov.au/nbscp/index.html) and the supply of IVIg in response to the recall was managed under this framework in an effective and timely way.
From the several options available to maintain supply, the NBA elected to authorise the use of additional domestic IVIg (Intragam) from CSL inventory, and to trigger the supply of Flebogamma by Lateral Diagnostics. Lateral Diagnostics was very responsive to the additional demand for product and met the supply requirement throughout the period of the Octagam recall.
Because the Blood Service is responsible for the authorisation and distribution of IVIg under the Deed of Agreement with the NBA, the Blood Service had a key role in supporting the increased use of Intragam and the transition to Flebogamma. The Blood Service worked with the NBA and the product suppliers to develop and circulate information to IVIg prescribers, and to manage the necessary logistics within the supply chain. Clinicians were able to switch their Octagam patients to alternative IVIg products with support from the Blood Service, and patients were able to continue their treatment with Intragram or Flebogamma with little, if any, disruption to their care.
International Consensus Conference on risk-based decision-making for blood safety
Blood safety decision-making has become increasingly complex with increasing expectation that aspects including scientific, medical, ethical, legal, regulatory, economic and public policy requirements should be taken into consideration. As blood systems globally focus on the responsible use of healthcare resources, questions arise on the most effective way to manage risk for blood safety that is sustainable. The focus on blood safety is increasingly considering risks beyond product safety towards a concept of ‘process integrity’—a ‘vein-to-vein’ system approach rather than a focus on ‘product’ alone.
In late October 2010 staff of the NBA attended an international consensus development conference on risk-based decision-making for blood safety that was held in Toronto. The intention of the conference was to seek recommendations and guidance on the creation of a framework for risk-based decision-making. The development of a risk framework for blood safety decision-making can build on knowledge relevant in the evaluation of previous blood safety decisions, haemovigilance data, models developed in other high risk disciplines and industries and leading practices in risk management, risk communication and systems thinking. An independent panel of 12 professionals with experience in the risk industry or health care developed the foundations of a vein-to-vein risk framework by answering pre-set questions designed by the organising committee.
The conference offered many valuable insights into the substantial costs and the very real challenges in developing a more comprehensive risk-based decision-making framework for the blood sector that would move the sector away from a precautionary approach to one based on a more holistic cost-benefit analysis.
The intent of the conference was discussed at the CTEPC Blood Policy forum in March by Adjunct Professor Chris Brook, who had participated on the independent panel. Future coordination of the policy framework and consideration of the issue by governments will be coordinated by DoHA. The NBA will assist in assessing the impact on the security of supply of any changes in the approach to risk for the sector.
SECTOR PERFORMANCE IMPROVEMENT
The NBA has a number of projects and activities designed to improve the overall efficiency of the sector as key strategies to improve affordability and minimise risk. These fall into three broad areas of improvement through:
- increasing capacity in data collection and analysis
- knowledge acquisition and management
- management and accountability initiatives.
Sector improvement through data collection and analysis
The availability of comprehensive, consistent, relevant, timely and robust data, and the capacity to analyse that data, is a powerful tool to identify areas where improvements can be made in supply management and clinical demand. During the year significant progress was made in the capture and analysis of data.
Implementation of BloodNet
Initially known as ORBS, the web-based ordering and receipting system for blood and blood products was developed by Queensland Health. The first site went live in January 2008 and was Queensland-wide by December 2008. During 2010–11, the NBA and Queensland Health conducted a national proof of concept trial of the system. Following the successful completion of the trial, the JBC provided interest monies to implement the system throughout Australia and to undertake further development to provide additional capabilities. To date the system has been rolled out to all hospitals in Queensland, South Australia, Tasmania, the Northern Territory and three hospitals in Victoria. Jurisdictions expressed satisfaction with the advice and approach taken for the national implementation of BloodNet, and the nature of the reports they receive from the system.
BLOODNET: VALUE ADDING IN THE SUPPLY CHAIN

Parliamentary Secretary for Health and Ageing, the Hon Catherine King MP (right), was given a live demonstration of BloodNet while on a visit to Darwin by the Royal Darwin Hospital’s Senior Scientist Kelly Burns.
Australia is already reaping the benefits of the roll out of NBA’s BloodNet, Australia’s first national, online, blood ordering system.
‘The BloodNet system is easy to use and requires little training,’ said Sue Williams, Senior Scientist, Transfusion, Queensland Health.
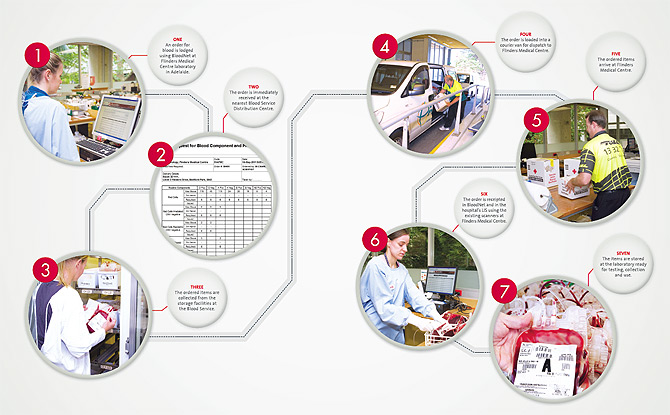
BloodNet replaces the manual ordering systems that relied on faxes and phone calls. Hospitals are now able to place an order for blood and blood products via a customised webpage. Once placed, the order is sent directly to the Blood Service by the click of a button.
The system is quick, easy and secure and the NBA is working with other blood product suppliers to enable them to be part of the online ordering system (see diagram overleaf).
BloodNet enables hospitals to:
- place orders 24 hours a day, 7 days a week and get instant confirmation that the order has been received
- give immediate feedback on receipt of orders, such as whether an item is damaged or close to the expiry date
- by late 2011 it will also allow for the recording and tracking of the fate of blood and blood products that are discarded or transferred to other hospitals.
BloodNet also provides data showing a hospital’s history of orders, use and trends over time as well as inventory levels and ordering practices. Hospitals can easily produce weekly, monthly or yearly reports on their blood use and obtain accurate financial details of their orders.
Implementation in private laboratories in Western Australia is scheduled for mid-July 2011, for the ACT and some area health networks in NSW in August and for the public facilities in Western Australia in September. Discussions with the remaining facilities in New South Wales and Victoria in relation to implementation are ongoing.
There is no cost to hospitals in introducing the system. The NBA is meeting all costs of implementing, supporting and further developing BloodNet.
BLOODNET IN 7 EASY STEPS
Australian Bleeding Disorders Registry
The ABDR is deployed to users in all Australian haemophilia treatment centres and clinics. The system is designed to produce data that fulfils the needs of clinicians, patient representative groups and governments within a highly controlled governance framework.
Substantial progress on increased data collection and quality was achieved during the year, with a noticeable increase in the quality of data, enabling the publishing of the first ABDR Annual Report (see www.nba.gov.au/abdr). Eighty-eight per cent of jurisdictional representatives expressed satisfaction with the data provided by the NBA from the ABDR.
The ABDR report is enabling us to compare Australian performance with other countries’ data. This will assist the NBA in planning appropriate long-term supply arrangements. An example of comparative data is shown in Table 3.15 below.
Planning work to further enhance the system commenced in June 2011.
NBA business intelligence system
Launched in January 2010, the NBA’s business intelligence system, known as Big Red, provides a single electronic repository for the secure storage, manipulation, integration and analysis of data drawn from other NBA systems.
During the last year, efforts on Big Red have been focussed primarily on data cleansing and integrating data from disparate systems into combined reports.
The technical work to enable secure remote access by jurisdictions to Big Red has been completed and this functionality will be deployed to jurisdictional users later in 2011 once work on the meta-data tool to accompany these data sets is completed. This is a key aspect of the delivery of the full functionality of BloodNet for JBC members allowing them to link data on demand and supply to compare ordering practices across AHPs.
Integrated data management system
The Integrated Data Management System (IDMS) is used by the NBA to manage the budgeting and forecasting of supply and demand for blood and blood products, inventory management and contract administration.
During 2010–11 a series of minor enhancements were undertaken to consolidate the functionality of the system in respect of demand planning and audit trails. Following these enhancements, use of the system continued to rise throughout the year with the system now an integral tool in daily work on contract administration and supply and demand planning.
Work is currently underway to electronically integrate the data from BloodNet relating to order and receipt verification into IDMS processes to validate supplier invoices for blood and blood products before payment. These system enhancements will be implemented in full during 2011–12.
The internal auditor conducted a review of the system including an assessment of risks, evaluation of controls, assessment of security, interfaces with other business systems and a general review of the performance of the system. The review confirmed the system’s functionality and its appropriateness as a platform for further development of NBA information and data strategies. A number of recommendations were made and the NBA is undertaking further analysis of several technical issues and refining how the system interacts with our business processes.
National IVIg management system
The National IVIg Management System (NIMS) will capture information on the use of IVIg in order to support and align with the Criteria for the clinical use of IVIg in Australia.
During 2010–11, the NBA planned the infrastructure needed for the system and the linkages between NIMS and other NBA sector systems such as BloodNet and ABDR, in preparation for the development and implementation of the system. Further development work was placed on hold following the decision of CTEPC to undertake an independent review of the management of IVIg (see pages 75–6).
Barcoding
The 2007 decision of the JBC to mandate specific standards for barcoding of blood and blood products has been subject to a further decision of the JBC to align the implementation of the policy with the implementation of blood ordering and receipting systems, made in May 2010.
The NBA will actively engage with the sector in 2011–12 to determine the specific details of implementation, including an agreed timetable.
Sector improvement through knowledge management
In depth knowledge of global trends, both in medical and pharmaceutical developments, in supply markets, and in how blood and blood products are managed elsewhere in the world, enable the NBA to provide high quality advice to governments and stakeholders, and to negotiate value for money contracts with its suppliers. Part 4 of this report, Horizon scanning, describes just some of the minor, major and simply interesting events that arose during the year. Other activities to build our knowledge base are described below.
The collaboration of national plasma product supply planners (NPPSpa)
The third meeting of the collaboration of national plasma product supply planners was held in Lisbon in March 2011, and chaired by the NBA’s General Manager. The group consists of a number of national agencies that have responsibility for plasma products. The aim of the group is to support participants’ needs for a secure, cost effective supply of plasma for fractionation, plasma derivatives and their clinically substitutable recombinant products. It continues to be the only international forum that shares data and experiences in the management of plasma products. Issues discussed included:
- patterns of IVIg usage, including sub-cutaneous Ig usage
- quantifying, attributing and funding mechanisms associated with the collection costs of plasma
- demand trends for clotting factors and other plasma products and the difficulty of predicting the budgetary impacts associated with the treatment of high cost patients
- international trade in plasma and IVIg products and supply, demand and price trends
- standards for plasma fractionation, especially in toll manufacturing agreements
- the use and cost effectiveness of solvent detergent pathogen inactivation to treat plasma products
- management of the availability of increasing numbers of product brands available in the haemophilia category, including the criteria for evaluating new proposals
- the ethical considerations associated with plasma collection.
The group agreed that the collaboration continues to be worthwhile and Canada was elected to chair the group in the future. During 2011, further comparative data will be assembled and analysed and the group will meet again in early 2012.
Other overseas intelligence gathering (see also page 60)
Two NBA officers visited the United Kingdom and the Republic of Ireland to assess relevant ICT and data systems to inform the further development of the NBA’s data activities. This included identifying any systems that could be purchased and used directly, either as a new capability or to replace an existing system with little modification. We were also interested to examine how the use of these systems had changed practice and product consumption.
The General Manager also visited the United Kingdom to:
- investigate operational aspects of blood sector management by the Welsh Blood Service, including inventory, distribution management, service standards and the impact of cost pressures. Of particular interest was the implementation of cell salvage throughout Wales
- explore the experience of the UK National Health Service (NHS) Commercial Medicines Unit in recent procurement of recombinant blood products and investigate the work of the NHS Better Blood Transfusion Program in developing models to predict future demand for red cells and platelets and in benchmarking and auditing safe and appropriate use of fresh blood components.
Staff who attended international conferences also took every opportunity to hold relevant side meetings with suppliers and colleagues in the blood sector from around the world. Key messages emerging from conferences attended are described below:
World Federation of Haemophilia XXIX International Congress
Evidence from around the world indicates that the demand for clotting factor support will continue to increase as a result of haemophilia populations in the developed world living longer and the introduction of more active prophylaxis treatment programs, some of life-long duration.
13th International Haemovigilance Seminar
International data points strongly to the need to focus resources around risks in the transfusion process, rather than implementing further measures to improve the safety of the blood products themselves.
International Plasma Protein Congress
A number of developments in IVIg of relevance to the Australian blood sector were reported including the introduction of an IVIg management program in the United Kingdom; an update on progress in clinical trials on the use of IVIg to treat Alzheimer’s disease; and increasing concerns about the incidence of thrombotic events with Immunoglobulin G.
Sector improvement through management and accountability initiatives
The NBA continued to review and assess policies and procedures across aspects of the blood supply chain, in order to increase efficiency and accountability within the blood sector, and internationally.
Performance measures for suppliers
The NBA continued to benchmark performance of contracts with suppliers for price and quality and also to provide reference points for sector performance improvement, as part of contract negotiations, see pages 46–55 & 57.
In particular, the extensive negotiations during the development of the new Deed of Agreement with the ARCS and implementation of output based funding, focused strongly on identifying opportunities to measure and improve performance, see pages 39–41.
Review of distribution arrangements for plasma and recombinant blood products
In 2010–11, stage 3 of the distribution review was implemented by the NBA. This required the NBA to undertake further evaluation of the manufacturer-direct distribution model to assess the costs and benefits for governments and the sector. A project board was established and distribution sites were visited. Key stakeholders were interviewed in Queensland, which agreed to be a site for a trial of the new arrangements.
The board’s findings were considered by the JBC in December 2010 which endorsed a two-year program to improve the supply chain for plasma and recombinant products, which would:
- develop an agreed set of commercially-based performance standards and information requirements for this supply chain
- monitor performance against these standards for all commercial suppliers and the Blood Service[1], and initiate improvements where appropriate
- explore opportunities for reducing the inventory of non-fresh products held by the Blood Service, using a risk-based assessment
- consolidate national direct-delivery arrangements for all clotting factors in conjunction with the further development of the ABDR.
AHCDO convened a workshop of haemophilia stakeholders in February 2011 to discuss opportunities and possible directions for supply chain improvements in relation to clotting factors. The outcomes of this discussion are assisting in framing specific options for further work.
Statement on national stewardship expectations for the supply of blood and blood products
The Statement on national stewardship expectations for the supply of blood and blood products has been developed to address the lack of specific accountability obligations, other than general safety and quality issues mandated by other agencies, on health providers such as laboratories in hospitals and clinics and other institutions that receive blood and blood products for dispensing to patients.
The Statement contains a concise description of responsible, sustainable and appropriate use of blood and blood products relevant to handling, storage, administration, usage and capacity to report inventory. It was developed following stakeholder consultation and advice from the JBC and was considered and endorsed by the AHMC on 12 November 2010. At the request of CTEPC, the NBA, in conjunction with JBC representatives, is currently developing a plan to promote and implement the Statement nationally, in conjunction with the ACSQHC National Safety and Quality Health Service Standard on blood and blood product.
Ensuring appropriate company behaviours
In January 2010 and January 2011 meetings were held in Dublin, in the Republic of Ireland, to draft a set of principles to promote the development of ethical and safe systems of blood and plasma collection and the manufacture of safe products for clinical treatment. Participants included both the industry and not-for-profit sectors, national blood authorities and patient and donor organisations. At the end of the initial meeting, a comprehensive statement—the Dublin Consensus Statement—was released which focused on the needs of patients and donors. During 2010 the Statement was published in Vox Sanguinis[2]; it has been endorsed by 22 patient groups and received qualified agreement from key global organisations.
At the second meeting the Statement was modified, with discussion around the issues of self sufficiency and a preferred focus on red blood cells and platelet donations. In particular, a number of organisations were concerned to ensure that the Statement should not specifically exclude the possibility of paying plasma donors. All of the participants agreed that the revised Statement was suitable for submitting to their organisations for formal endorsement. The Statement has not yet been endorsed by Australian governments or internationally.
The NBA has responded to the Statement by introducing additional requirements in its documentation to procure plasma-derived blood products. Companies tendering to the NBA for these contracts must now demonstrate that they and their plasma suppliers maintain ethical policies and practices in relation to patients, blood and plasma donors, sector relationships and global use of donated blood and plasma. The new specification is consistent with Commonwealth procurement guidelines and the National Blood Agreement.
Performance scorecard for the sector
Key performance indicators are essential tools for both monitoring and improving the quality of health services. Significant advances have been made over the past decade in the development of indicators for the Australian health sector, focusing mainly in the field of acute hospital care. As a result, the language and culture of performance measurement is now well established in the day-to-day life of acute public hospitals.
Ongoing development of performance measures is occurring on multiple fronts as part of Australian Government and state and territory initiatives that aim to establish an information infrastructure at the service delivery level. These are designed to:
- support and encourage good practice
- regularly inform about consumer outcomes
- inform judgments about value for money
- produce national and state and territory data on performance to enhance accountability.
The National Blood Agreement requires the NBA to:
- undertake or facilitate national information management, benchmarking and cost and performance evaluation for the national blood supply (paragraph 25(o) of the National Blood Agreement)
- facilitate the development of national information systems for safety and quality issues in relation to the Australian blood sector (paragraph 35(f) of the National Blood Agreement).
The NBA is developing a framework that will allow a set of key performance indicators, aligned with the national health performance framework, to be developed for use in benchmarking and monitoring the blood sector.
There are a number of measures that could be drawn from the blood sector to contribute to the overall understanding of the health status of the Australian population. For example, morbidity due to bleeding disorders and measures of the burden of disease for people with bleeding disorders would provide good insight into health outcomes for a defined population group. These may include years lived with disability, impairment rating, disability-adjusted life expectancy, years of life lost and cause of death.
Community capacity to meet the needs of people with bleeding disorders such as the distance to a haemophilia treatment centre, the rate of prophylaxis and the rates of haemophilia could be measured in the ‘determinants of health’ domain. The rate of occurrence of haemophilia was used as an example of an appropriate indicator for this domain in the initial report of the National Health Performance Committee. Data is now available for these types of measures to facilitate international comparisons.
The central tasks for this project are to interpret how the national health system performance framework aligns with types of information within the blood sector, and propose indicators for adoption at the national level. There are three types of information for the blood sector:
- management—focusing on demonstrating achievement of the objectives of governments, including value for money
- clinical—focusing on patient outcomes and safety and quality of processes used to deliver these outcomes. The clinical domain comprises measures of the impact of health care on a patient and indicators of this type are often described as the ‘gold standard’ of service effectiveness indicators
- supply—focusing on the degree to which suppliers to the sector are fulfilling the expectations of governments in the most efficient and effective manner.
During 2011–12, the draft measures will be further developed to reflect data that is currently available or will be available over time. A discussion paper will then be circulated to the sector for comment.
[1] As a distributor: the Blood Service is responsible for delivering some blood products other than fresh blood components.
[2] Mahony BO, Turner A, “The Dublin Consensus Statement on vital issues relating to the collection of blood and plasma and the manufacture of plasma products” Vox Sanguinis 2010 98,447-450