National Blood Authority Australia
Annual Report 2010–11
Part 3: PERFORMANCE
3.4 APPROPRIATE PATIENT BLOOD MANAGEMENT AND SAFE USE OF BLOOD AND BLOOD PRODUCTS
The NBA contributes to promoting the safe, high quality management and use of blood and blood products and services by working with stakeholders and other experts to develop clinical practice and product use guidelines that support effective and appropriate clinical practice.
In 2010–11 key areas of work included:
- national patient blood management guidelines and initiatives
- review of the criteria for the clinical use of IVIg
- national haemovigilance program
- National Safety and Quality Health Service Standard for blood and blood product
- red cell data usage project
- education initiatives.
| Key performance indicators | Measure | Results | |
| Quality advice provided to guide promotion of safe, high quality patient blood management and use of blood and blood products | High level of satisfaction of the NBA’s advice on the promotion of patient blood management and use of blood and blood products as assessed through a survey of JBC | 88% of the jurisdictions were satisfied with the NBA’s advice on the process undertaken to develop patient blood management guidelines and the IVIg criteria, noting the need for further evaluation of cost-effectiveness. (Pages 71–5) |
|
| Numbers of downloads of guidelines and criteria from NBA website (www.nba.gov.au ) | Guidelines–329 Criteria–253 (Pages 71–5) |
||
| Deliverables | Measure | Results | |
| Provide clinicians with evidence-based information on safe and appropriate blood management by releasing two elements of the National Health and Medical Research Council Clinical Practice Guidelines for Patient Blood Management | Two guidelines released by 30 June 2011 | PBM Guideline module on Critical Bleeding/Massive Transfusion released on 31 March 2011 PBM Guideline module on Perioperative public consultation process closed on 1 April 2011 (Pages 71–3) |
PATIENT BLOOD MANAGEMENT GUIDELINES AND INITIATIVES
Internationally, patient blood management is a well-established approach which aims to avoid unnecessary transfusion and the associated risks, and to optimise the use of donor blood. The NBA has developed a wide-ranging program to implement these objectives, which includes updating guidelines, developing national outcome and performance measures, and supporting the development and national roll-out of successful educational initiatives.
Clinical practice guidelines for patient blood management
Using interest monies approved by the JBC, the NBA is managing the development of evidence-based Patient Blood Management Guidelines. The interest monies support the costs of systematic reviewers, publication and promulgation of the guidelines, and clinical meetings. The NBA procures and manages contractors, liaises with all government agencies including the NHMRC, coordinates all project related activities including clinical meetings, and conducts extensive quality assurance of technical reports and the guidelines throughout all stages of development.

Members of the Clinical Reference Group for the Perioperative PBM Module: Dr Hilary Cadman, Dr Suzanne Campbell, Dr Craig French, Associate Professor Larry McNicol, Ms Tracy Merlin, Dr Richard Seigne, Mr Daryl Teague and Dr Amanda Thomson, with NBA staff Dr Chris Hogan, Dr Paul Hyland, Ms Jennifer Roberts and Ms Leia Earnshaw. Missing were Professor Zsolt Balogh, Mr Shannon Farmer, Professor Russell Gruen and Dr John Vinen
The guidelines will comprise a set of six separate modules that are being developed sequentially and will focus on different patient populations: Critical Bleeding/Massive Transfusion; Perioperative; Medical; Critical Care; Obstetrics; and Paediatrics/Neonates. NHMRC approval will be sought on completion of each module. Combined, the suite of six modules will replace the NHMRC/Australian and New Zealand Society for Blood Transfusion, Guideline on the use of blood components (2001).
In 2009–10 the systematic reviews and public consultation processes were completed for both the Critical Bleeding/Massive Transfusion and Perioperative Modules. After peer review and an independent AGREE II assessment, the Critical Bleeding/Massive Transfusion module was approved by NHMRC on 12 November 2010 and released on 31 March 2011.
Public consultation for the Perioperative Module closed on the 1st April 2011 with 25 submissions being received. The module is expected to be finalised for independent peer review and Agree II assessment in July prior to consideration by the NHMRC at its meeting in October 2011.
The systematic review and writing of the Medical and Critical Care modules are progressing and are expected to be released for public consultation early in 2012.
NEW ADVICE TO GUIDE CLINICAL PRACTICE
The release of the revised Critical Bleeding/Massive Transfusion module on 31 March 2011 represented the culmination of nearly three years of work on the part of the NBA and its knowledge network of clinical specialists and practitioners.
‘I believe this is an important body of work and the Society is pleased to continue to have the opportunity to be involved in guideline development with the Authority,’ said Associate Professor Michael O’Leary of the Australian and New Zealand Intensive Care Society.
Publication of the module in 2009–10 was delayed due to the complexity and volume of material generated by the review and its release now heralds significant improvements in the evidence for, and quality of, patient blood management standards.
The previous protocols recommended transfusion of a large number of red cells, which assisted in replacing blood loss but did not take into account the need to stop the blood loss. The information in the new module consolidates evidence that supports more comprehensive management of critical bleeding incidents and promotes local adaptation and use of a massive transfusion protocol.
The module is one of six that together will form the Clinical Practice Guidelines for Patient Blood Management. They will replace the NHMRC/Australian and New Zealand Society for Blood Transfusion, Guideline on the use of blood components (2001).
Dr Bronwen Ross, Deputy CEO, Royal College of Pathologists of Australasia acknowledged ‘...the significant work that has gone into producing this document. The College appreciates the opportunity to be involved in the development of these guidelines...’
The Critical Bleeding/Massive Transfusion module sets out the material in a logical way and is currently available in both electronic and print format, free of charge. Copies can be obtained or ordered from the NBA website at http://www.nba.gov.au/guidelines/module1/index.html.
Demand for the module has been high. In order to make the information more accessible to health practitioners, the development of an iPad/iPhone application for the modules of the Patient Blood Management Guideline is underway.


National patient blood management initiatives
Internationally, hospitals that have introduced patient blood management (PBM) practices have reported significant health care savings and improved patient outcomes. In Western Australia, where initial steps have been made to introduce PBM practices, a reduced demand for red cells has been found in the pilot institution. The NBA established a National Patient Blood Management Steering Committee (NPBMSC) in 2009 to provide advice to governments on patient blood management initiatives.
In providing advice, the NPBMSC referred to the research emerging from the systematic review being conducted to support the production of the patient blood management guideline modules. The NPBMSC has proposed a set of national outcome and performance measures and the development of a Patient Blood Management Toolkit to support the introduction of patient blood management at the local level.
The measures and toolkit concepts were considered by JBC and the CTEPC in March 2011. Work on the toolkit will commence early in 2011–12.
Key achievements include:
- definition of anaemia management priorities by the Anaemia Management Working Group, a sub-group of the NPBMSC including:
- engagement of the Royal College of Pathologists Australia to standardise information included in pathology reports relating to anaemia
- engagement of the National Prescribing Service to include anaemia in their educational offerings
- liaison with the TGA on issues relating to availability of iron products
- incorporation of PBM content into education programs supported by the JBC including the BloodSafe eLearning Program and Melbourne University’s Post Graduate Certificate in Transfusion Practice
- contribution to the DoHA Review of the Funding of Pathology Services in Australia to encourage appropriate stewardship of blood and blood products
- provision of detailed guidance to, and sector coordination of, input on all aspects of the National Safety and Quality Health Service Standard for blood and blood product.
CRITERIA FOR THE CLINICAL USE OF INTRAVENOUS IMMUNOGLOBULIN
IVIg is used to replace or modify a person’s immune response. It is used to treat many different indications across immunology, neurology, haematology and other specialty areas, for these two purposes. Many of the indications for which IVIg is used are extremely rare, and in these circumstances, evidence of IVIg efficacy is limited.
Review of the Criteria for the clinical use of IVIg in Australia (the Criteria)
The Criteria identifies the indications for which IVIg is funded under the national blood arrangements by all Australian governments. Regular review of the Criteria is required to align funded access to IVIg with the latest evidence, or in the case of limited evidence, a consensus of expert opinion.
A triennial review of the Criteria was conducted by the National IVIg Criteria Review Working Group (NICRWG) established and supported by the NBA. The working group received and considered 28 formal submissions in 2010 and a systematic review was conducted for indications where sufficient evidence was available.
An exposure draft was developed and approved by the NICRWG and the JBC for public consultation and released on 25 June 2011 for a period of eight weeks. The NICRWG will consider the feedback prior to submission of the revisions through the JBC to health ministers for approval.
It is anticipated that the second edition of the Criteria will be finalised and released on 1 July 2012.
Review of the management of IVIg
In response to concerns at the need for a higher level of cost effectiveness to be applied to the use of IVIg, the NBA, working with the government members of the NICRWG, commenced an analysis of the existing management arrangements for IVIg and alternatives that could be applied.
This analysis showed that in 2009–10, the cost of subsidised IVIg was $275 million, which represented 31per cent of the blood budget. In 2003–04, this cost was $134 million. Both figures include the cost of plasma collected by the Blood Service for the locally manufactured product. Between the two periods, the average cost per patient increased by 25 per cent from approximately $20,000 to $25,000. In the period 2003–04 and 2007–08, prior to the release of the Criteria, growth in IVIg usage was approximately 14 per cent per annum.
Since then, growth has averaged 11.5 per cent per annum. While contemporary data on the amount and cost of treating chronic versus acute diseases with IVIg are not readily available, a number of observations can be made. For example, certain conditions such as primary immunodeficiency disease (PID) which accounts for about 17 per cent of total IVIg issued, requires IVIg for life, commencing in early childhood. For a typical adult PID patient the cost per year for IVIg is approximately $30,000. The biggest change in demand from 2004–05 to 2009–10 has been for the treatment of conditions where there is convincing evidence of benefit; see Part 4, pages 101–3.
The range of indications for which IVIg therapy demonstrates some clinical benefit is expanding, with the product’s immunomodulatory effects being the current mode of action of greatest interest. For example, Baxter International Inc. is currently undertaking multi-centre trials on the use of IVIg in the management of Alzheimer’s disease. The potential for a significant and ongoing growth in demand coupled with an ever expanding list of indications poses challenges for both supply security and affordability. The key challenge in determining the cost effectiveness of IVIg for other conditions, which affect only small patient numbers, is the lack of supporting data.
Preliminary analysis has found that the current authorisation arrangements are not consistent across jurisdictions. They differ in their focus on the public and private sectors and the extent to which there is a dedicated review of individual patients on IVIg treatment to determine efficacy. The analysis also noted that there is no national peak body for reporting and analysing trends.
In May 2011, CTEPC determined that the NBA, with the JBC, should coordinate an independent review of IVIg management arrangements, with the following goals:
- ensuring that funded IVIg use reflects best clinical practice and is cost effective
- ensuring that the outcomes of decision-making regarding access to IVIg funded under the national blood arrangements are consistent with the current Criteria
- improving the capture of information on the effectiveness of IVIg usage to improve the evidence base that will inform future changes as to what is regarded as best practice in IVIg use and prescribing.
Work on this project will commence in August 2011.
NATIONAL HAEMOVIGILANCE PROGRAM
The transfusion of blood and blood products is a core component for healthcare service delivery to patients. However, the transfusion of blood products is not without risk and can lead to complications. The monitoring of serious adverse events resulting from transfusion is critical to transfusion safety. These systems are known as haemovigilance systems.
The World Health Organization Global Database on Blood Safety Report 2004–05 indicates that 42 of 105 reporting countries had a national haemovigilance system in place and 24 were in the process of developing one.
Working within the NBA’s Haemovigilance Program, the Haemovigilance Advisory Committee (HAC), established in 2009, contributes to improving patient outcomes by providing enhanced and nationally consistent reporting on transfusion-related adverse events at a national level.
In 2010, HAC published the second Australian Haemovigilance Report, http://www.nba.gov.au/haemovigilance/index.html based on the agreed National Haemovigilance Data Dictionary. The report collated 294 serious adverse incidents reported between July 2008 and June 2009 and made 12 key recommendations in the areas of data quality, procedural errors and national blood quality and safety initiatives.
During the year HAC met to consider a strategy for addressing Recommendation 2 of the 2010 Haemovigilance report on improving reporting and recognition of serious adverse events such as transfusion-associated circulatory overload (TACO) and transfusion related acute lung injury (TRALI). See Figure 3.23 for images of TRALI-affected and recovered patient chest x-rays.
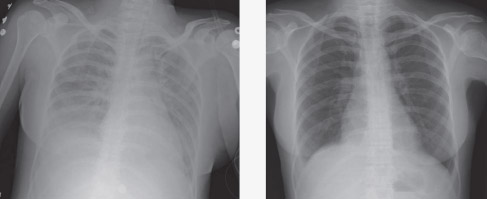
FIGURE 3.23 TRALI: chest x-ray findings (a) at the time of acute symptoms and (b) after weaning from a ventilator (Transfusion related acute lung injury presenting with acute dyspnoea: a case report. Journal of Medical Case Reports 2008, 2:336. doi:10.1186/1752-1947-2-336)
HAC agreed that a consensus guideline on the Recognition and Management of Acute Transfusion Related Adverse Events should be developed and that this document should include sections on TACO and TRALI. The NBA has worked with the HAC out of session to develop the structure of the guideline and will seek assistance from an expert society to draft the content of the report.
National Safety and Quality Health Service Standard for blood and blood product
The NBA contributed to the development of the draft National Safety and Quality Health Service Standard for blood and blood product. The standard has been developed by the ACSQHC after consideration of the NBA’s request to include a blood specific standard in their suite of 10 key standards. The NBA has assisted the ACSQHC in consulting widely with the blood sector over the past three years in the development of this important Standard.
The Standard is built around four key elements:
- the governance and quality improvement systems—health services should have in place systems that are safe and minimise waste at all stages of the provision, use, storage and distribution of blood and blood products
- the documentation of patient information—the clinical workforce should accurately record a patient’s blood and blood product use including transfusion history and indications for treatment
- communicating with patients—the clinical workforce should inform patients and carers about the options and risks for any treatment plan that may include the use of blood and blood products
- blood and blood product management processes—health services should have systems in place to obtain, store, prescribe, transport and administer blood appropriately, efficiently and safely.
The draft standard was made available for a period of public consultation, which ended on 8 October 2010. The NBA provided two formal submissions to the ACSQHC; one to the public consultation process and another to inform the estimate of regulatory impacts associated with implementing the national standards and accreditation framework.
The new Standard is expected to be made available during 2011 for full implementation from January 2013. The NBA will continue to work closely with the ACSQHC to ensure that we communicate the principles of the Statement on national stewardship expectations for the supply of blood and blood products (see page 68) in alignment with this Standard.
RED BLOOD CELL USAGE PROJECT
The use of red blood cell products has been shown to be highly variable, with some patients receiving transfusions that are not required. Although jurisdictions have safety and quality programs aimed at improving the rate of clinically appropriate transfusions, these programs have limited access to data on blood use drivers and trends.
The purpose of the National Data Linkage Project is to facilitate jurisdictional-based data linkage with the aim of producing a nationally consistent dataset of, and ultimately a national report on, blood product use in Australia.
The diagram in Figure 3.24 illustrates how the data linkage will be implemented.
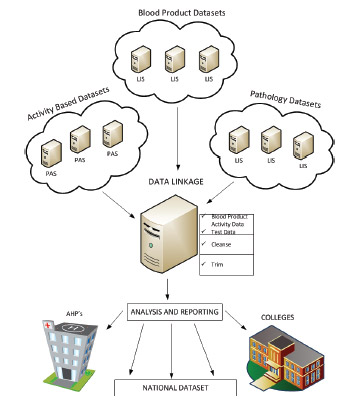
FIGURE 3.24 Red cell data linkage datasets
During 2010–11 good progress was made towards this aim with the completion of a detailed methodology and most states and territories confirming the scope of activity required to achieve this data linkage.
The data linkage project is expected to deliver three major benefits for the sector namely:
- improved understanding of blood product use and trends generally
- improved information to support clinical practice improvements
- improved information to support compliance with current and future sector standards.
The project is focused on providing data as shown in Table 3.16.
Notes:
SRG—Service-related group
DRG—Diagnosis-related group
ICD—International classification of diseases
A workshop has been scheduled for early August 2011 to determine how best to report and present the data at a local and state level and to determine which data to consolidate into a national report. It is expected that a national report on red cell data usage, and to some extent other fresh product usage, will be produced by June 2012.
EDUCATION INITIATIVES
The NBA considers education initiatives an important tool in implementing patient blood management and improving the safety, quality and efficiency of the clinical use of blood and blood products. Using interest monies approved by the JBC, the NBA has facilitated and supported the development of local programs to make them available nationally.
Bloodsafe e-learning Australia
In 2006, the South Australian Department of Health funded the development of an online education package for clinical staff involved in the transfusion chain. Now available nationally, the Clinical Transfusion Practice course available on the BloodSafe e-Learning Australia website continues to be very popular throughout Australia with over 90,000 people having registered for the program up to the end of June 2011, and a completion rate of approximately 74 per cent.
Interest in the e-Learning website has continued throughout the year, with an average of at least 2,500 user registrations per month. Nurses are the largest users at around 90 per cent of all registrations. User support and evaluation data demonstrate high levels of user satisfaction.
During 2010–11, work continued on development of new courses for ‘postpartum haemorrhage’ and ‘iron deficiency anaemia’, which will supplement the existing Clinical Transfusion Practice course. These are expected to be available by October and December 2011 respectively.
In addition, strategies were developed to evaluate the BloodSafe e-Learning Australia website and to promote its use.
Graduate program in transfusion practice
The NBA entered into an agreement with the Victorian Department of Health to revise the Graduate Certificate in Transfusion Practice to update the course content to incorporate key patient blood management concepts. The update was completed and the revised course content has been implemented.
The aim of the course is to develop and educate health professionals who have roles in improving transfusion safety and appropriateness of use of blood and blood products within hospitals. The foundation of the work is the translation of national best practice guidelines into every day transfusion practice. It is offered as a four subject online course, facilitated by an experienced online nurse educator. The course is endorsed by the Blood Matters program of the Victorian Department of Health, and the Blood Service. This is the only graduate e-certificate online blood transfusion course in the southern hemisphere.