You are here
Objective 2. Improve risk management and blood sector performance
In 2016-17, the NBA achieved a range of objectives to improve blood sector performance and risk management, particularly in the areas of Ig governance, evaluation of new products, ICT developments, data availability and analysis and risk and knowledge management.
Ig Governance
The NBA Ig Governance program was very active during 2016-17. The activities within the program continue to improve the governance and management of publicly funded Ig to ensure product use and management:
- reflects appropriate clinical practice
- represents efficient, effective and ethical expenditure of government funds
- is consistent with relevant national safety and quality standards for health care.
During the 2016-17 period, the program focussed on the four key activities listed below:
- Launch of BloodSTAR (Blood System for Tracking Authorisations and Reviews)
- Publication of the National Policy: Access to Government Funded Immunoglobulin Products in Australia 2nd edition (National Policy)
- Publication of Module 2 of Managing Blood and Blood Product Inventory: Guidelines for Australian Health Providers - Managing Intravenous and Subcutaneous Immunoglobulin Inventory (Guidelines for Managing Blood and Blood Product Inventory)
- Review of the Criteria for the clinical use of intravenous immunoglobulin in Australia (the Criteria).
To support this work, three meetings of the National Immunoglobulin Governance Advisory Committee (NIGAC) were convened.
Launch of BloodSTAR
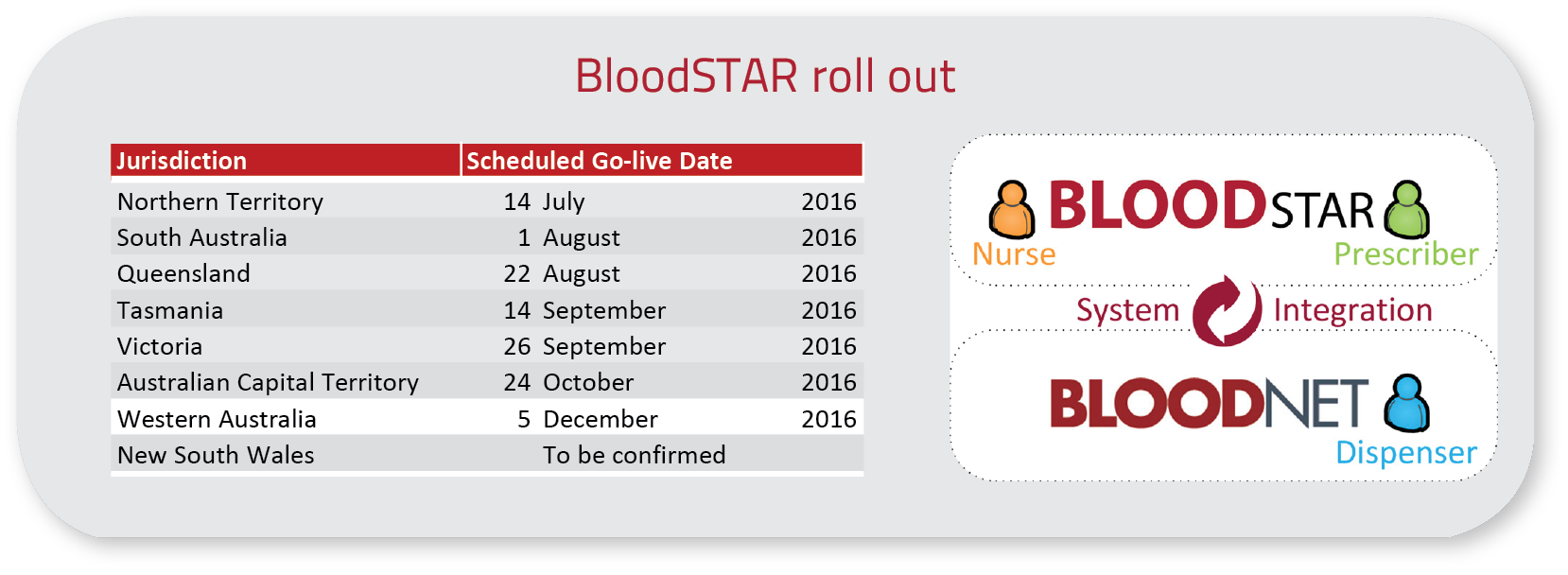
During 2016-17, the NBA successfully launched BloodSTAR in all states except New South Wales. This included the seamless transition of 6,868 existing patients to the new system ensuring continuity in health care.
The NBA developed the new online system on behalf of all Australian Governments to support health providers in managing their Ig Governance obligations as set out in the National Policy. System capabilities support prescribers, nurses/midwives, dispensers and authorisers to deliver high quality care to patients across Australia. The system standardises and manages access to the supply of immunoglobulin products by enabling authorisation requests to be submitted electronically and work-flowed to an authoriser for assessment and approval. Authorisation requests can only be approved for funding by all governments through the national blood arrangements, for conditions identified in the Criteria. BloodSTAR will also enable collection of improved national data and enhance the ability to further develop the Criteria and provide an improved evidence base for practice improvement and research.
Publication of the second edition of the National Policy
The second edition of the National Policy was released in July 2016 to coincide with the launch of BloodSTAR. The document describes the authorisation arrangements for access to government-funded immunoglobulin products. This includes an explanation of roles, responsibilities, authority and accountability of those involved in requesting authorisation, authorising, supplying, managing and using immunoglobulin products throughout the supply chain within health services. The second edition of the National Policy incorporates business processes associated with BloodSTAR. It replaces the first edition of the Ig Governance National Policy November (released in 2014) in jurisdictions using BloodSTAR. The document underwent public consultation prior to release.
Publication of Module 2 of the Guidelines for Managing Blood and Blood Product Inventory
The Guidelines for Managing Blood and Blood Product Inventory provide better practice processes that can be used by health providers to ensure risks associated with receipt, storage, collection and transport of blood and blood products are mitigated. It also identifies improvement opportunities for implementation.
In 2016-17, the NBA developed Module 2 to supplement the overarching inventory management principles and support the implementation of BloodSTAR. The module aims to assist health providers in meeting the requirements of the National Policy by:
- describing how to establish and manage stock levels
- outlining the Ig product ordering models
- identifying different methods to determine ordering requirements/triggers
- providing recommendations for good practice.
The document underwent public consultation prior to release.
Blood System for Tracking Authorisations and Reviews (BloodSTAR) - Rollout
BloodSTAR was developed by the National Blood Authority (NBA) and first implemented in July 2016 as one of the key measures under the National Immunoglobulin (Ig) Governance Program. BloodSTAR was developed to improve the governance and management of government funded Ig to ensure that product use and management reflects appropriate clinical practice and represents efficient, effective and ethical expenditure of government funds.
BloodSTAR was rolled out across Australia via a staged state by state process. The NBA worked closely with each jurisdiction and relevant health sector stakeholders to map out key business process changes and to communicate these widely. The NBA and the Australian Red Cross Blood Service managed the transition from the predominantly paper based processes. Education and training on the system was provided via a series of face to face and online training sessions. Consent to record personal and sensitive information in BloodSTAR was obtained from patients who were authorised to receive Ig product beyond their state 'go live' date. Information was gathered on relationships between treating, administering and dispensing facilities so they could be matched and entered correctly to ensure continuity of care and a smooth transition.
BloodSTAR is now live in all states and territories except for NSW and manages all patients who are authorised under the Criteria for the Clinical Use of Intravenous Immunoglobulin (IVIg) in Australia to receive government funded Ig. There are currently over 7,700 patients with active authorisations and 6,800 users accessing the system as either, authorisers, medical officers, nurses, admin support officers or facility administrators.
The key benefits of BloodSTAR include ensuring consistency of processes and application of the Criteria, and transparency of information enabling support for patients across multiple sites. BloodSTAR will facilitate all future improvements and further development of the Criteria to ensure government funded Ig is directed to where there is evidence of greatest benefit.
Review of the Criteria
The Criteria assists clinicians and transfusion medicine professionals to identify the conditions and circumstances for which immunoglobulin products are available for use under the national blood arrangements. The review of the Criteria continued in 2016-17. Work completed as part of this activity supports the Criteria to more clearly articulate and standardise the diagnostic, qualifying and review criteria, initial and continuing authorisation periods, dosing controls and ensure the submission of supporting evidence for access to funded Ig.
In 2016-17, Specialist Working Groups for Neurology, Immunology, Haematology and Transplantation Medicine assisted further progression of the review for medical conditions in chapters 7 (Exceptional therapeutic use) and 8 (Not supported) of the Criteria with a view to implementation in BloodSTAR. Public consultation was also undertaken to seek community wide feedback on the proposed revisions to the medical conditions included in chapters 7 and 8.
Adaptation of the Criteria for BloodSTAR
BloodSTAR (Blood System for Tracking Authorisations and Reviews) is the new online system developed by the National Blood Authority on behalf of all Australian Governments to support health providers in managing their Ig Governance obligations as set out in the National Policy: Access to Government Funded Immunoglobulin Products in Australia(National Policy). The system standardises and manages access to the supply of immunoglobulin products for the treatment of conditions identified in the Criteria for the clinical use of intravenous immunoglobulin in Australia (the Criteria), funded by all governments through the national blood arrangements.
With the move to this new online system, the Criteria will no longer be published in printed form. The Criteria that has been uploaded into BloodSTAR has been based on an adaptation of the Second Edition (or Version 2) of the hard copy publication and the previous paper authorisation request forms. The adaptation has been required because there were certain fields in the system that could not be populated directly from Version 2 of the Criteria either because they were absent or ambiguous, or only referred to indirectly.
This is particularly the case for review criteria. In many instances, to allow prescribers to conduct a review within BloodSTAR, there must be an initial qualifying value, which is entered at the initial authorisation request. Where review or other criteria were inferred in Version 2 and the detail was available from Version 3 the information from Version 3, has been used to populate the required fields in BloodSTAR.
The evidence items fields are available in the system for the following reasons:
- To capture data or values (such as platelet count or Ig level) that are currently collected on the paper request forms
- To clarify qualifying criteria where the published version is silent or ambiguous (such as words like thrombocytopenia or recurrent)
- To assist prescribers with baseline values they may like to compare at review
- To assist in data analysis to inform future criteria access arrangements or development of the system.

Evaluation of new products
The working group established by the Jurisdictional Blood Committee (JBC), including NBA and jurisdictional representatives, progressed work on the requirements and processes for evaluations to be undertaken under Schedule 4 of the National Blood Agreement.
The NBA undertook an evaluation of a Schedule 4 proposal for the inclusion of C1 Esterase Inhibitor Concentrate as a new product under the national blood arrangements. The Medical Services Advisory Committee (MSAC) provided advice on the proposal on 30 July 2015.
In late December 2015 the JBC agreed to recommend the inclusion of C1 Esterase Inhibitor Concentrate in the National Products and Services List and the 2016-17 NSP&B for Type I or II hereditary angioedema for the following indications:
- treatment of acute attacks
- pre-procedural (short term) prophylaxis for high risk procedures such as dental work, head or neck surgery, or surgery requiring intubation
- routine (long term) prophylaxis for patients who experience the equivalent of eight or more acute attacks per month.
The NBA worked with the Australasian Society of Clinical Immunology and Allergy (ASCIA) to develop guidance and governance arrangements for use of C1 Esterase Inhibitor Concentrate for the clinical circumstances which reflect JBC's approval.
The NSP&B was subsequently approved by health ministers in April 2016. Supply of C1 Esterase Inhibitor Concentrate commenced in October 2016.
Data developments
In 2016-17, the NBA continued to build its data capture and analysis capabilities across all aspects of the supply chain. This area of activity is a key strategy to improve the overall efficiency and sustainability of the sector by providing a measurement for improvement.
A significant amount of data and information exists within the blood sector, however, the extent to which this data is currently available to the parties that need it, the quality of the data, and the capacity of the systems that hold it, varies widely. During 2016-17, the NBA progressed the following activities identified in the National Blood Sector Data and Information Strategy and Scorecard 2013-2016:
- continued to develop the list of system reports to be provided to stakeholders and developed specifications to assist in their development
- refined and implemented monthly and quarterly issue reports to be provided to stakeholders
- discard data
- collected, analysed and distributed discard data from the BloodNet Fate Module to support the establishment of revised targets for discard rates in 2018-19 under the National Blood and Blood Product Wastage Reduction Strategy 2013-2017
- specified further BloodNet discard reports on red blood cell ABO groups and reporting by public and private health providers
- Haemovigilance
- developed the National Haemovigilance Report 2017 based on data for 2014-15 collected by states and territories. Refined and implemented the work plan to support implementation of the Strategic Framework for the National Haemovigilance Program approved in 2014-15
- continued to develop revised haemovigilance tools and templates to support haemovigilance programs in Queensland
- published the standards and minimum data sets for haemovigilance reporting for implementation in 2017-18
- Australian Bleeding Disorders Registry (ABDR)
- published the ABDR Annual Report for 2015-16
- continued to develop the set of data standards as part of the data integrity process for the ABDR for review by AHCDO Executive and the Data Managers
- provided to AHCDO the 2015-16 ABDR Benchmarking Report
- the NBA signed Information Framework Agreements with SA in 2014-15, with NT, TAS and WA in 2015-16 and NSW in 2016-17. These agreements are required as part of the National Blood Sector Data and Information Governance Framework
- published the Ig Annual Report for 2015-16
- responded to 71 data requests from internal and external stakeholders.
Risk management
Focussing on risk management remained a high priority in 2016-17 to ensure a safe and secure blood supply within Australia. As part of that focus, the NBA commenced updating several programs during 2016-17 including:
- review and update of the NBA Risk Management Policy and Framework was undertaken and is expected to be endorsed by the Chief Executive in August 2017
- revision and testing of supply risk mitigation arrangements for imported products
- review and update of the NBA business contingency arrangements.
National Blood Supply Contingency Plan (NBSCP)
The NBSCP remains a body of work that will go to the Jurisdictional Blood Committee for endorsement in December 2017.
Recent changes have been made to the suite of documents including:
- expanded information covering roles and responsibilities
- communication channels in times of activation of the plan
- escalation and management responsibilities during an activation of the plan.
Business Contingency Plan (BCP)
The NBA commenced implementing the recommendations from an internal audit of the NBA Business Contingency Plan (BCP) during 2016-17. The BCP was seen as a detailed and robust approach to contingency planning. However, given the NBA has introduced several new programs recently, it was recommended a business impact analysis be carried out to review the suite of core business processes presently defined in the plan.
Review of Risk Management in the Blood Sector
The NBA continued to progress a range of recommendations resulting from an independent comprehensive Review of Risk Management in the Blood Sector, previously known as the National Managed Fund (NMF) Action Plan Review. This included approval of strategic level recommendations by all health ministers through the COAG Health Council. The Review concluded that the overall level of risk in the blood sector has reduced over the last decade due to a range of factors, including supply security improvements undertaken by the NBA. The negotiation between the NBA and the Australian Red Cross Society for the new Deed of Agreement implemented on 1 July 2016 addressed a number of these recommendations, together with a range of operational improvements for implementation in consultation with the Blood Service.
Supply Risk Mitigation for Plasma Derived and Recombinant Products
In 2016-17, the NBA conducted a specific risk simulation exercise to test the existing risk mitigation strategies in place under the National Blood Supply Contingency Plan (NBSCP). The risk exercise was conducted with an imported products supplier to simulate an interruption to product supply, testing the effectiveness of responses in a controlled environment. Lessons learned from the simulation exercise were used to update and improve the NBA's approach to risk management and assist in preparation for future activations of the NBSCP.
Information Communication Technology (ICT)
The NBA ICT teams continue to provide technology solutions and high quality support for NBA staff and deliver approved JBC strategies and projects. These projects are a key enabler of both data collection/analysis and business process reform across the sector. This is mainly incorporated under the Crimson Portfolio. The Crimson Portfolio incorporates the JBC approved Blood Sector Systems 2016 to 2019 project as well as BloodNet laboratory information system (LIS) integration stage 2 program and will deliver the following high level outcomes:
- policy compliance
- infrastructure updates
- platform enhancements
- reporting capability
- ABDR and MyABDR enhancements
- BloodNet enhancements
- BloodSTAR enhancements
- BloodNet-LIS interface.
BloodNet-LIS interface - Pathology North's (NSW) LIS eBlood went live in October 2016. It is the first ever BloodNet-LIS developed and certified according to the newest specifications. Pathology Queensland (QLD) successfully implemented the first ever BloodNet-AUSLAB LIS interface going live in June 2017. All BloodNet-LIS interfaces currently in place are responsible for processing 35 per cent of total national issues of fresh blood products.
Australian Bleeding Disorders Registry (ABDR)
The ABDR and associated patient portal (MyABDR) is a clinical tool used on a daily basis by clinicians in all Australian haemophilia treatment centres to assist in the management and treatment of people with bleeding disorders. The NBA delivered a number of updates and improvements in 2016-17 to enhance the functionality and user experience with both ABDR and MyABDR. These changes included:
- system wide use of time zone processing which was particularly important for patients entering in MyABDR data
- updates to treatment plans
- additional requested validation messages and instructions as requested by users
- assorted user-requested functionality updates.
ICT Crimson
Crimson is a Jurisdictional Blood Committee approved project spanning over three years from 2016 to 2019. The aim of the project is to enhance Blood Sector Systems to continue to provide clinical, data, accountability and supply chain resilience and efficiency benefits to the NBA and across the blood sector.
The project also aims to ensure NBA systems are compliant with government policies and standards, including the Digital Transformation Agencies' Digital Service Standard. Through this, solutions are designed for users and feedback is sought regularly, improving the engagement of stakeholders and usability of systems. This ensures greater uptake and usage and improves accuracy and completeness of data collection for the benefit of all jurisdictions and the NBA.
The Crimson project will develop and implement a range of improvements, including:
- streamlining of all user interfaces
- enhanced integration of modules and whole systems
- introduction of real time notifications
- improvements or development of reports
- ability to support all new barcoding formats
- enhancements to platforms and infrastructure.
Crimson incorporates the BloodNet 5 project - the redevelopment of the BloodNet system, and a vast array of improvements to the Australian Bleeding Disorders Registry (ABDR), MyABDR and BloodSTAR.
BloodNet 5 prototype image
BloodNet 5 prototype image
BloodNet
The BloodNet 5 redevelopment project commenced in late August 2016 and included a number of NBA ICT staff visiting laboratories across the country to gain exposure to real users and environments. In March 2017, BloodNet 5 passed the government's Digital Service Standard alpha assessment highlighting the successful approach to ensuring the new system is designed around end user needs. BloodNet 5 is currently scheduled for implementation in early 2018 and is progressing well.
The BloodNet Authorisation module was developed in 2016-17 enabling an interface between BloodNet and BloodSTAR to ensure that medical officers and nurses using BloodSTAR have consistent, up-to-date data from dispenses in BloodNet. Face to face and online training for this module were provided to each jurisdiction in conjunction with the national implementation of the BloodSTAR system.
The BloodNet User Reference Group met regularly throughout the year and provided feedback on how the authorisation module was working within laboratories after implementation. The NBA has responded to feedback and provided further updates to ensure the system is usable and suitable for all users.
In June 2017, QLD Health Auslab LIS interface with BloodNet was successfully established.
The BloodNet User Reference Group met regularly throughout the year to provide feedback to the NBA.

Image: BloodNet User Reference Group
BloodNet Redevelopment
BloodNet is Australia's online blood ordering and inventory management system. BloodNet provides the ability for Australian hospitals and laboratories to order blood and blood products
and is supported by a 24-hour service to ensure that essential, lifesaving products get to where they are needed. Over one million orders have been placed in BloodNet since late 2010 and approximately 28,000 litres of blood are ordered and tracked each month.
BloodNet transformation
In 2009 the Queensland Department of Health developed an online system for ordering and tracking blood products called ORBS (Ordering, Receipting Blood System). This system was so successful that it was proposed for the NBA to develop a national system. A bespoke application called BloodNet was developed by the NBA in 2010 and has continued to be enhanced since this time.
In 2016, the NBA recognised the significant opportunity of redeveloping BloodNet to achieve compliance with the government's Digital Service Standard and received the Jurisdictional Blood Committee's authorisation to commission the BloodNet 5 project. The project will deliver a new BloodNet designed around users to make ordering blood products easier and faster and to build a more resilient and feature rich system.
The NBA visited 138 people across 39 different locations around Australia in order to gather feedback from users to ensure the new system meets their needs. The BloodNet User Reference Group was established to ensure feedback is discussed and progress on the system can be demonstrated at regular times through the project.
The redevelopment will come to fruition with the uptake by all health providers.

BloodNet Milestone at Fiona Stanley Hospital, Western Australia
On 11 November 2016 PathWest Fiona Stanley Hospital Laboratory placed the one millionth order of blood product in the BloodNet system. The National Blood Authority and the Western Australian Department of Health visited the Fiona Stanley Hospital to celebrate this significant event. A commemorative award was presented by the NBA Chief Executive, Mr John Cahill to the PathWest Fiona Stanley Hospital Laboratory Senior Scientist, Ms Annette Le-Viellez.
The event in Perth highlighted the significant and critical role of BloodNet across the blood sector since it was rolled out across Western Australia.

2016-17 Sector monitoring
In 2016-17, the NBA continued its horizon scanning of international experience that may influence the management of blood and blood products in Australia. This monitoring activity informs the provision of current and proactive analysis to governments to enable the NBA to fulfil its functions under the National Blood Agreement.
Our focus in 2016-17 was:
- new product developments and applications
- global regulatory and blood practice trends
- scientific and clinical research with implications for future supply or demand in the sector
- business events that may have an impact on global supply, demand and pricing, such as changes in company structure, financial outlook, production capacity, organisation, ownership, and marketing and contractual arrangements
- diseases or pandemics that may have an impact on supply or risks to the safety of products
- developments in testing methods, vaccines and disease control strategies that could potentially mitigate risks to supply
- any other emerging risks that could potentially put financial or other pressures of any kind on the Australian sector.
The NBA regularly posts to its website a selection of items from this horizon scanning process, illustrating the wide range of factors which may influence industry operations and patient outcomes. This information is available from www.blood.gov.au/monitoring-international-trends-blood-sector.
During 2016-17, key developments included:
- increased availability of longer acting recombinant clotting factors for patient convenience
- further progress on an investigational humanised bispecific monoclonal antibody engineered to mimic the function of factor VIII, and simultaneously bind factors IXa and X
- the spin out of Biogen's global haemophilia business as a new public company, Bioverativ
- research of variable success on gene therapies directed at disorders such as haemophilia,
sickle-cell disease and beta thalassemia, and continued interest in the treatment of haemophilia with RNAi therapeutics - progress in developing treatment to reduce sickle-cell related pain crises
- further clinical trials of plasma-derived alpha-1 antitrypsin in a variety of settings
- introduction of new treatments for hereditary angioedema
- new haemostasis products
- interest in a variety of ways of storing/preserving blood and blood products
- further research on whether the age of red blood cells transfused affected clinical outcomes
- continuing endorsement of a restrictive transfusion policy, as the body of clinical experience grows in a range of surgical contexts
- increased acceptance of the benefits of treating anaemia pre-operatively
- more emphasis on maintaining iron levels in blood donors
- further clinical experience of novel anticoagulants and reversing their effects when required
- further stem cell research relevant to the blood sector, both on producing transfusible product and on modifying the surgical contexts in which transfusion may be considered
- the development of an implant that resembles natural bone, with working marrow capable of producing blood
- further understanding of how long the Zika virus remains in the human body and can be transmitted to others
- continuing reports of cases of Middle East Respiratory Syndrome-Coronavirus (MERS-CoV), primarily in Saudi Arabia, with further confirmation that camels are a source of infection and hospital clusters are not uncommon
- continuing reports of human cases of avian influenza A (H7N9) by the Chinese mainland health authorities, and further confirmation that live poultry markets are a major source of human infection. While temporary poultry market closures reduced environmental levels of H7N9 and other avian flu viruses, contamination returned to previous levels when markets re-opened.
