
In this Winter 2019 issue:
- BloodNet System – Update
- BloodNet Milestones
- Laboratory Information System (LIS) Interface Updates
- InterSystems TrakCare BloodNet LIS Vendor Certification
- Pathology Queensland LIS-BloodNet-BloodSTAR Interface Pilot Release Success
- User Tip – Special Orders
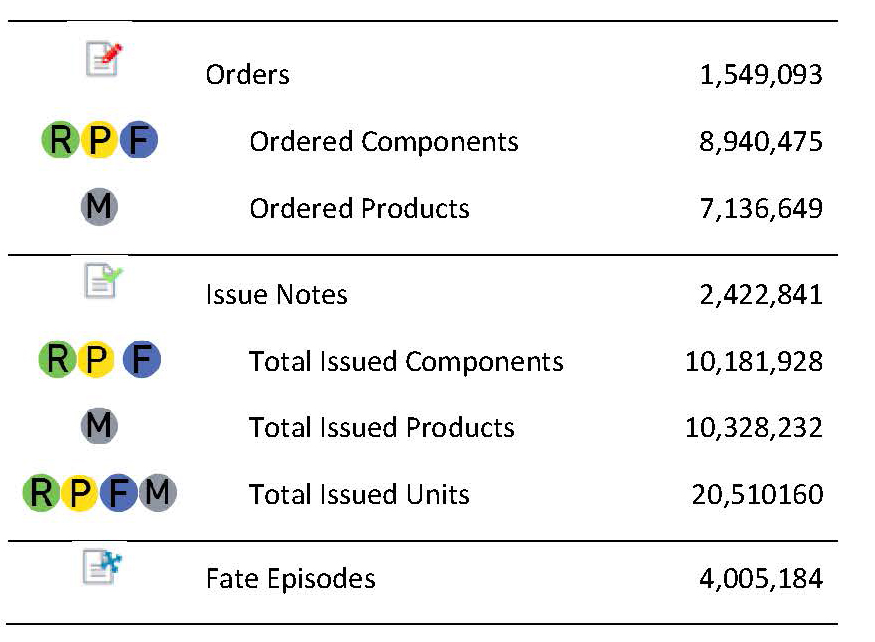
BloodNet Milestones!
The 1.5 millionth BloodNet order was placed on 17th April by Western Diagnostic Pathology, Peel Campus in Western Australia. Congratulations!Here is the latest update to our amazing statistics from the last newsletter noting we have now issued over 20 million products and components since the start of BloodNet!!
Laboratory Information System (LIS) Interface Updates
Currently, several of the National Blood Authority funded LIS Vendors have completed, or are nearing completion of, BloodNet LIS Vendor Certification. Those already certified have interfaced with BloodNet, or are now working with Facilities to make interfacing a reality.
Once a LIS is Vendor Certified it is possible for any Facility using a certified LIS to undertake local Facility Certification. Once approved, this will allow for release into the Facility’s Production environments.
If you would like to reap the rewards that BloodNet LIS interfacing provides, please contact your LIS provider or the NBA (support@blood.gov.au or 13 000 BLOOD) to begin this process.
National BloodNET LIS Vendors Interface Status Update
| LIS | Interface Status |
| Apollo (Sonic Healthcare) | Awaiting quote from vendor |
| AUSLAB (Citadel Health) | In production |
| e-Blood(Citadel Health) | In production |
| BloodTrack (Haemonetics) | Available for implementation |
| MediPATH (LRS Health) | Awaiting quote from vendor |
| Millenium PathNet (Cerner) | Currently under development |
| Pathology Laboratory System (Kestral) | Currently under development |
| SoftBank (SCC Soft Computer) | Currently under development |
| Sunquest Blood Bank (Sunquest Information Systems) | Currently under development |
| TrakCare Laboratory (Intersystems) | Available for implementation |
| Ultra (Cirdan) | Available for implementation |
For any information regarding BloodNet LIS Interfacing or to contact the National Blood Authority please click here.
InterSystems TrakCare BloodNet LIS Vendor Certification
InterSystems TrakCare Laboratory Information System, made possible through National Blood Authority funding in April 2016, achieved Vendor Certification on 08 January 2019. This has enabled TrakCare BloodNet LIS interface implementations to be progressed nation-wide.
With BloodNet already interfaced to eBlood (Pathology North, NSW), AUSLAB (Pathology Queensland, QLD) and Cerner Millennium (Sydney South West Pathology Service, NSW), the news that laboratories using TrakCare LIS will soon be able to interface directly with BloodNet will be welcomed by many.
InterSystems have already begun action to support all users of TrakCare LIS to achieve implementation.
![]() This includes:
This includes:
- Northern Territory Health, NT
- Launceston General Hospital and surrounding areas, TAS
- St Vincent’s Hospital, Sydney, NSW
- St Vincent’s Hospital, VIC
- Goulburn Valley Health, VIC
“Laboratories that use TrakCare currently process approximately 4% of total blood supplies nationally and these laboratories are looking forward to the benefits a BloodNet LIS interface brings.
InterSystems, now Vendor Certified, have engaged with all their users to support them in seeking Facility Certification. This will enable implementation into the Facility’s Production environments” said Nathan Kruger, BloodNet LIS SME and Assistant Director with the National Blood Authority.
Laboratories with a BloodNet-LIS interface have identified significant time savings, with major metropolitan laboratories reporting:
- Time saving for laboratory staff of 17.5 hours per week;
- Improved data accuracy; and
- Process efficiencies with the new linkage to BloodSTAR.
Pathology services who currently use TrakCare LIS can discuss implementation plans for a BloodNet-TrakCare interface with their InterSystems representative.
Pathology Queensland LIS-BloodNet-BloodSTAR Interface Pilot Release Success
Citadel Health (AUSLAB) achieved BloodNet LIS Vendor Certification on 17 May 2017. This enabled Queensland Health to achieve BloodNet LIS Facility Certification, which went live on 7 June 2017. This implementation accounts for 11% of national orders and over 10% of IVIg dispenses nationally.
In early 2018, Pathology Queensland and the NBA piloted the integration of BloodSTAR through the AUSLAB-BloodNet LIS interface in conjunction with six laboratories of varying sizes.
This pilot validated the benefits associated with the dispense and management of IVIg supply through successful patient authorisation matching, time savings, reduction in handling times and improved accuracy in real time of IVIg inventory levels in the Queensland public health system.
In January 2019, after further BloodNet LIS refinements following the BloodNet 5 upgrade, Queensland Pathology rolled out the full AUSLAB-BloodNet-BloodSTAR LIS interface to all 34 sites, updating BloodSTAR patient authorisations directly from their LIS.
‘A BloodNet interface is in use at all 34 Pathology Qld sites. In March 2018, a trial at 6 sites allowed fate messages to also update BloodSTAR dispense episodes. We found the interface worked well for routine dispense episodes, which meant staff did not have to ‘double handle’ and dispense in BloodNet in addition to AUSLAB.’ said Sue Williams, Supervising Scientist, Transfusion, Central Laboratory.
‘This was a significant time saver as each dispense episode would take 2-3min and in our hospital, this would equate to close to 1 hour of laboratory time/ week.’
This is already showing benefits by automatically matching patient authorisations over 87% of the time for the 18,057 IVIg dispenses in their laboratories. This translates to 15,710 automatically matchedpatient authorisations in BloodSTAR from AUSLAB via the BloodNet LIS interface!
For the remaining 13% of patient authorisations that don’t match automatically, some human intervention is required to determine the patient’s correct authorisation. This occurs when patients have more than one authorisation, or where there has been a substantial change in treatment planning. These processes have been streamlined in BloodNet and can be resolved through the far simpler unmatched episodes and discrepancies functions. Using either of these functions in BloodNet takes a fraction of the time enabling staff to focus on other important tasks.
This integration is providing laboratories and the NBA real time data streams for IVIg dispense and return LIS actions. The resulting fate messages automatically sent when products are dispensed from the laboratory, ensure that dispense and return to stock practices are recorded and compliant with IVIg governance for the authorisation and batch product details. These benefits are evident in feedback the NBA has received following the pilot:
- ’Pathology Qld laboratories dispensed approximately 48,000 bottles of intravenous or subcutaneous immunoglobulin in 2018. This interface saves valuable staff time as around 45,000 would be fated automatically.’
- ‘Patients who have complicated dose schedules do need to be monitored and may need manual intervention.’
- ‘This was especially helpful for our busy sole operator shift workers, especially in the outlying regions, who dispense product early in the morning, so the product is ready for clinics.’
This innovative and successful integration would not be possible without the vision, energy, great work and substantial effort that Sue Williams and the Pathology Queensland team have given to the Pilot.
Through the early adoption of an integration solution, their valuable contributions and support have delivered the benefits of integration and paved the way for all future LIS-BloodNet-LIS interface implementations to include BloodSTAR integration.
The NBA wishes to thank Pathology Queensland for its involvement. It has been another wonderful collaboration that has enabled the first ever LIS-BloodNet-BloodSTAR Interface release.
User Tip – Special Orders
Special Orders are placed when a fresh product is:
- required to have specific modifier(s),
- has antigen requirements, or
- if there is a need for it to be provided for a specific patient.
Special orders should not be used for routine stock orders. Facility Administrators can add and/or edit missing products to your facilities stock order template if required. If you have a recurring order which meets the special order requirements, you can save this order as a ‘Facility favourite’ for regular use.
For further information
Further information on BloodNet is available online at https://www.blood.gov.au/bloodnet or by contacting the NBA on 13 000 BLOOD (13 000 25663) or support@blood.gov.au

