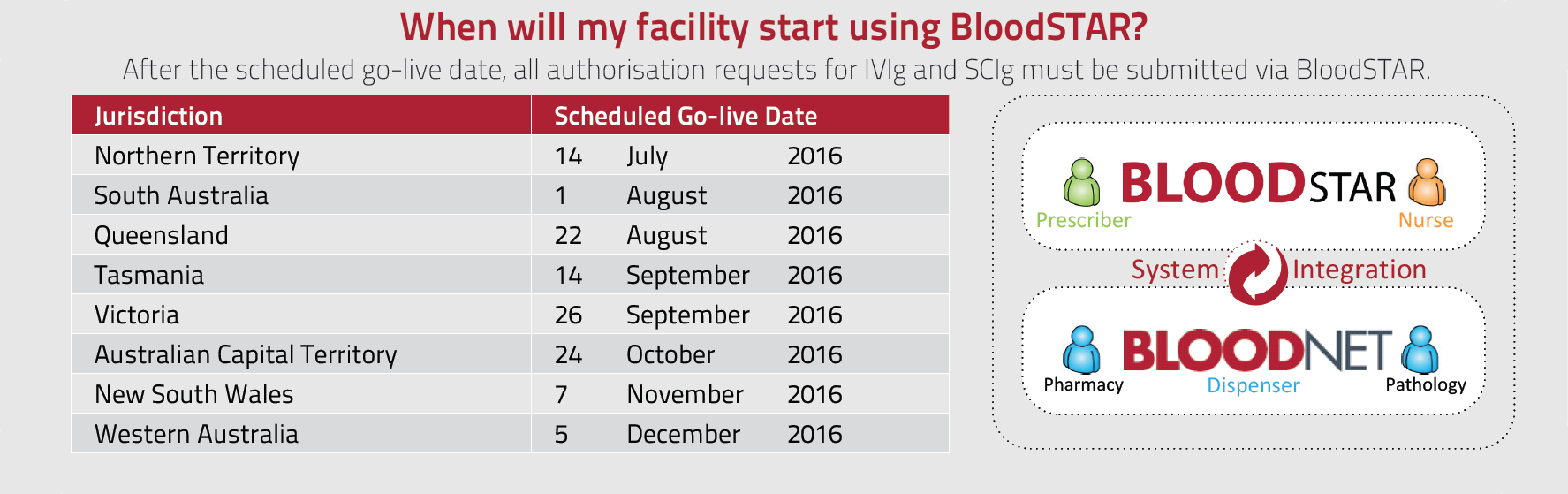
As we move closer to the go live dates for BloodSTAR, the National Blood Authority (NBA) will be providing users with a monthly update on the BloodSTAR system development and transition activities. Each monthly issue will highlight key activities and upcoming timeframes for system readiness. Please forward this on to any colleagues that it may be relevant to.
Over the next two months, the Newsletters will focus on the following topics:
April – User registration, system training schedule and support materials.
May – User registration reminder and how to get training before your go live.
If there are other topics that you would like us to address, please email your suggestions to support@blood.gov.au.
In This Issue:
- What will BloodSTAR mean for me?
- Important process changes for BloodSTAR go live
- Final Call – Register your facility and facility administrators*
- ACTION REQUIRED – What is required now?
- What is happening next?
- Download BloodSTAR News
 (pdf) (181.96 KB) or
(pdf) (181.96 KB) or  (docx) (158.7 KB)
(docx) (158.7 KB)
What will BloodSTAR mean for me?
BloodSTAR will have impacts on current business processes for all health care professionals involved in the management of patients and facilities receiving Immunoglobulin (Ig) products. Documents outlining these key changes and checklists of actions required for each key user group are available on the NBA website at http://www.blood.gov.au/bloodstar-support-materials.
Actions required include:
Prescribers - ensure privacy consent for each of your patients has been provided to the Australian Red Cross Lifeblood (Lifeblood),
- ensure that all facilities at which you work are registered for BloodSTAR,
- register yourself for BloodSTAR from 4 April 2016 onwards,
- discuss the dispense request processes with your dispense facility,
- be aware of your go live date, and
- read and understand the Ig product prescriber roles and responsibilities within the Ig Governance National Policy currently open for public consultation at http://www.blood.gov.au/public-consultation.
Nurses - register yourself for BloodSTAR from 4 April 2016 onwards,
- discuss the dispense request processes with your dispense facility,
- be aware of your go live date, and
- read and understand the Ig product nurse roles and responsibilities within the Ig Governance National Policy currently open for public consultation at http://www.blood.gov.au/public-consultation.
Dispensers - discuss with your colleagues and agree on a process with each facility you dispense to on how you want to receive your dispense requests,
- read the Ig product dispenser roles and responsibilities within the Ig Governance National Policy currently open for public consultation at http://www.blood.gov.au/public-consultation, and
- update your BloodNet stock order template to include relevant Ig products as future Ig orders will be placed as normal stock. For further information please see Ig Inventory Management Guidelines currently open for public consultation at http://www.blood.gov.au/public-consultation.
For more details on these points please refer to the documents ‘What does BloodSTAR mean for me?’ on the NBA website.
Important process changes at BloodSTAR go live
There will be process change requirements at go live of BloodSTAR that users must be aware of and implement. These will be outlined in detail before go live, some key changes include:
- A successful authorisation approval for a patient WILL NOT automatically trigger an Ig product dispense request. All requests for Ig, including the first dose, will need to be forwarded to your dispenser NOT Lifeblood. Lifeblood will no longer be faxing dispense requests and/or authorisation approvals to either the treatment or dispensing facilities.
- It will be the responsibility of the dispenser to check the authorisation of the patient and ensure they order and maintain adequate Ig stock to meet dispense requests for authorised patients, this will no longer be done by Lifeblood.
Final Call – Register your facility and facility administrators*
All facilities that prescribe and manage patients receiving Ig product must register their facility for BloodSTAR. Facilities should be registered by 4 April 2016 to ensure individual users are able to register against that facility. NSW public facilities and staff should refer to the note below re specific arrangements for them.
To register your facility please go to http://www.blood.gov.au/registering-your-facility-bloodstar and complete the registration form.
If you are unable to register your facility by this date please contact the NBA support team on 13 000 BLOOD (13 000 25663) and we will assist you with your registration.
Dispensers will not need to register for BloodSTAR as they will access a new Authorisation module in BloodNet to manage product ordering, check authorisations, dispense product for authorised patients and perform reconciliation. If you currently dispense Ig and DO NOT have access to BloodNet please contact 13 000 BLOOD (13 000 25663).
|
BloodSTAR Classification |
Facility Type |
Facility Functions |
Does my facility need to register for BloodSTAR? |
|
Treatment Facility |
Clinic |
Ig Prescribing and/or |
Yes - |
| Doctor's rooms |
Ig Patient Management and/or |
||
| Hospital | Infusion of Ig Product | ||
|
Dispensing Facility |
Pathology |
Receive Ig Stock and/or |
No – |
| Pharmacy | Dispense Ig to Patients |
*NSW public facilities and staff please note: NSW public facilities and staff (prescribers, nurses and dispensers) are not required to register until further notice. The NBA is working directly with NSW Health to develop suitable arrangements for the state-wide implementation of BloodSTAR. The NBA is responsible for the implementing BloodSTAR in the private sector in NSW so private facilities and staff (prescribers, nurses and dispensers) should follow the instructions provided by the NBA.
ACTION REQUIRED - What is required now?
- Last opportunity to register your facility before user registration opens!
Facility Registration is essential to ensure adequate time for your facility to be set up in BloodSTAR by April 2016. It is also a pre-requisite for:
- User registration - Prescribers and Nurses who practice within your facility cannot register for access to BloodSTAR until it is registered.
- Transition of existing Ig patients - Patients attending your facility who are authorised for ongoing Ig treatment cannot be entered into BloodSTAR by Lifeblood prior to go-live (July – December 2016, depending on your jurisdiction) unless the facility and their treating medical specialist have been registered in the system.
- Nominate your facility administrators – You will need to nominate at least two facility administrators to approve all user registrations for your facility.
Register now at http://www.blood.gov.au/registering-your-facility-bloodstar.
- Read and understand the process changes that must occur for your role and at your facility before your BloodSTAR go live date.
Read the documents and action the check list for your role at http://www.blood.gov.au/bloodstar-support-materials.
 I'm a Dispenser...What does BloodSTAR mean for me? (pdf) (489.57 KB)
I'm a Dispenser...What does BloodSTAR mean for me? (pdf) (489.57 KB) I'm a Dispenser...What does BloodSTAR mean for me? (docx) (422.9 KB)
I'm a Dispenser...What does BloodSTAR mean for me? (docx) (422.9 KB) I'm a Nurse....What does BloodSTAR mean for me? (pdf) (379.93 KB)
I'm a Nurse....What does BloodSTAR mean for me? (pdf) (379.93 KB) I'm a Nurse....What does BloodSTAR mean for me? (docx) (425.57 KB)
I'm a Nurse....What does BloodSTAR mean for me? (docx) (425.57 KB) I'm a Prescriber...What does BloodSTAR mean for me? (pdf) (436.45 KB)
I'm a Prescriber...What does BloodSTAR mean for me? (pdf) (436.45 KB) I'm a Prescriber...What does BloodSTAR mean for me? (docx) (425.75 KB)
I'm a Prescriber...What does BloodSTAR mean for me? (docx) (425.75 KB)
What is happening next?
User Acceptance Testing Feedback
Thank you to those who participated in the final User Acceptance Testing (UAT) of BloodSTAR from 9 to 22 March 2016. The NBA will be analysing feedback received and will prepare communications to inform you of any changes that have come out of the UAT process and address any queries raised.
BloodSTAR User Registration - OPEN 4 April 2016
Individual user registration opened on Monday 4 April 2016.
While this is early for some State/Territories, prescribers and nurses must register as soon as possible after 4 April 2016.
If you are a Treating Medical Specialist, your currently authorised patients will only be entered into BloodSTAR for you if;
- you register early for BloodSTAR,
- your patients have provided consent (and a copy has been provided to Lifeblood)
- your patient/s have authorisation beyond the go live date, and
- your patient’s treating facility is registered for BloodSTAR.
Reminders and To-Do-Lists
- Facility registrations must be completed now so that information can be entered into BloodSTAR about your facility before user registration opens on 4 April 2016.
- Be familiar with the changes relevant to you – read the documents and action the check list for your role. Documents available at http://www.blood.gov.au/bloodstar-support-materials.
- Sign up for this newsletter - create a BloodPortal account at https://portal.blood.gov.au and from the home page click on ‘My Subscriptions’ and ‘Subscribe’ to Immunoglobulin.
Further information on BloodSTAR is available online at http://www.blood.gov.au/bloodstar or by contacting 13 000 BLOOD (13 000 25663) or support@blood.gov.au.