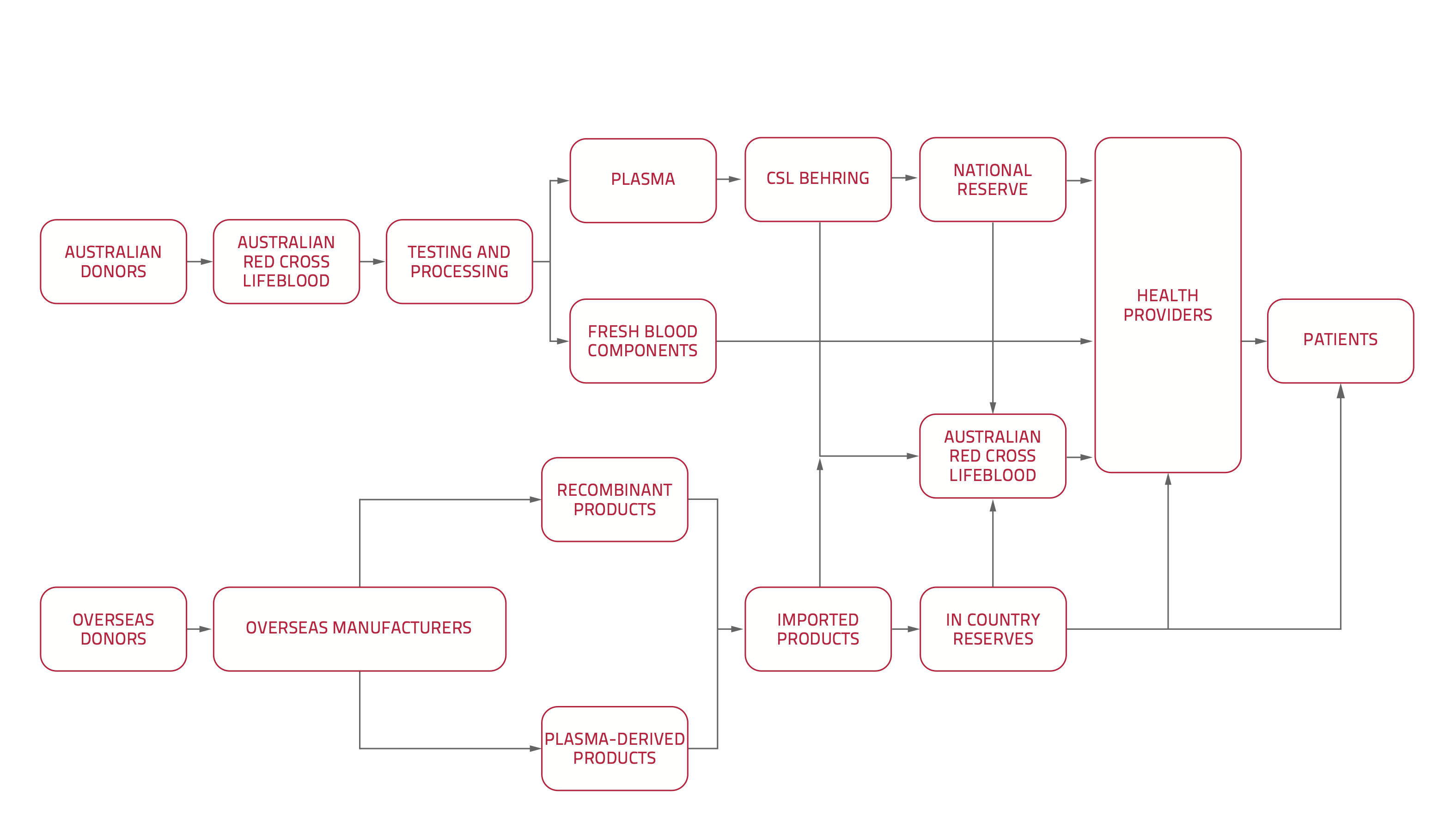
Australia's national blood supply system
We manage and coordinate arrangements for the supply of blood, blood products and blood services in Australia.
Under the National Blood Agreement, our role is to deliver an uninterrupted supply of blood and blood products to Australians in need.
Our work:
- secures the supply of fresh blood and other blood products
- improves risk management and blood sector performance
- promotes the safe and efficient use of blood and blood products.
Supply of blood and blood products is managed under the national blood arrangements, which are a national scheme for funding blood products. They are similar to other national health subsidy schemes, such as:
- Medicare
- the Pharmaceutical Benefits Scheme
- My Aged Care
- the Hearing Services Program
- the National Diabetes Services Scheme.
The national blood supply chain differs from other national subsidy schemes in the following ways:
- It has a shared funding framework where the Australian Government provides 63% of all funding and the states and territories provide the other 37%.
- All governments agree on policy aims and objectives under the National Blood Agreement.
- The subsidy is delivered through centralised supply contracts that we agree and administer within the Australian Government procurement and financial accountability framework.
- Blood and blood products are provided at no cost to patients in Australia.
Supply planning
Under the National Blood Agreement, we manage annual supply and production plans and budgets. We consult on these with our suppliers and the Australian, state and territory governments (the jurisdictions).
We collaborate with the jurisdictions to develop an annual National Supply Plan and Budget (NSP&B) for the supply and demand of blood products and services for Australian Health Providers (AHP). The process includes preparing the National Product Price List for publicly funded blood and blood products. We are responsible for procuring the products that health ministers agree to make available.
We use information from our suppliers, IT systems such as BloodNet, and the states and territories to forecast product volumes required for the next financial year. Every January we review the NSP&B mid-year figures against the full-year budget, and adjust our planning accordingly. At the end of the financial year, we complete a full reconciliation process. This ensures the jurisdictions only pay for products they have received, rather than their forecast demand.
Managing the blood supply
We work with Lifeblood and other suppliers to secure Australia's supply of blood, blood products and blood services.
To fulfil our requirements under the National Blood Agreement, we monitor the balance between supply and demand across the country throughout the year. This includes intensive management of products with limited supply to ensure that treatments are available for Australian patients.
Products we fund
We are responsible for procuring the products that health ministers agree to make available. Publicly funded products are included in the National Product Price List(Opens in a new tab/window).
Find out more about publicly funded blood products in Australia.
Accessing and ordering blood products
We use several information and communications technology systems to help us provide a safe, secure and affordable blood supply for all Australians. We call these our Blood Sector Systems.
Health professionals can use these Blood Sector Systems to manage product orders, patient treatments, inventory and reporting.
With BloodPortal, one username and password gives you access to all of our Blood Sector Systems, including:
- Australian Bleeding Disorders Registry
- BloodNet
- BloodSTAR
- Jurisdictional Reports.
There are also several governance arrangements in place to ensure products are ordered and used appropriately. Find out more about accessing and ordering blood products.
Get in touch
To find out more about blood products supply, please contact us.
Last updated: 27 Mar 2024
